David A. O'Connell, Ricki S. Carroll, Angela L. Duker, Andrea J. Schelhaas, Marjorie M. Postell, Paul T. Fawcett, Michael B. Bober
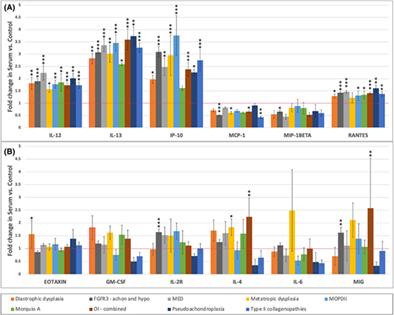
{"title":"小儿骨骼发育不良的血清细胞因子表达趋势","authors":"David A. O'Connell, Ricki S. Carroll, Angela L. Duker, Andrea J. Schelhaas, Marjorie M. Postell, Paul T. Fawcett, Michael B. Bober","doi":"10.1002/jbm4.10816","DOIUrl":null,"url":null,"abstract":"<p>The skeletal dysplasias are a heterogeneous group of genetic conditions caused by abnormalities of growth, development, and maintenance of bone and cartilage. Little is known about the roles that cytokines play in the inflammatory and non-inflammatory pathophysiology of skeletal dysplasia. We sought to test our hypothesis that cytokines would be differentially expressed in children with skeletal dysplasia as compared to typically growing controls. Cytokine levels were analyzed using the Cytokine Human Magnetic 25-Plex Panel (Invitrogen, Waltham, MA, USA); 136 growing individuals with skeletal dysplasia and compared to a cohort of 275 healthy pediatric control subjects. We focused on the expression of 12 cytokines across nine dysplasia cohorts. The most common skeletal dysplasia diagnoses were: achondroplasia (58), osteogenesis imperfecta (19), type II collagenopathies (11), multiple epiphyseal dysplasia (MED: 9), diastrophic dysplasia (8), metatropic dysplasia (8), and microcephalic osteodysplastic primordial dwarfism type II (MOPDII: 8). Of the 108 specific observations made, 45 (41.7%) demonstrated statistically significant differences of expression between controls and individuals with skeletal dysplasia. Four of the 12 analyzed cytokines demonstrated elevated expression above control levels in all of the dysplasia cohorts (interleukin 12 [IL-12], IL-13, interferon γ-induced protein 10 kDa [IP-10], regulated on activation, normal T cell expressed and secreted [RANTES]) and two demonstrated expression below control levels across all dysplasia cohorts (monocyte chemoattractant protein 1 [MCP-1], macrophage inflammatory protein-1β [MIP-1β]). The highest levels of overexpression were seen in MOPDII, with expression levels of IP-10 being increased 3.8-fold (<i>p</i> < 0.0001). The lowest statistically significant levels of expressions were in type II collagenopathies, with expression levels of MCP-1 being expressed 0.43-fold lower (<i>p</i> < 0.005). With this data, we hope to lay the groundwork for future directions in dysplasia research that will enhance our understanding of these complex signaling pathways. Looking forward, validating these early trends in cytokine expression, and associating the observed variations with trends in the progression of dysplasia may offer new candidates for clinical biomarkers or even new therapeutics. © 2023 The Authors. <i>JBMR Plus</i> published by Wiley Periodicals LLC. on behalf of American Society for Bone and Mineral Research.</p>","PeriodicalId":14611,"journal":{"name":"JBMR Plus","volume":"7 12","pages":""},"PeriodicalIF":3.4000,"publicationDate":"2023-11-09","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://asbmr.onlinelibrary.wiley.com/doi/epdf/10.1002/jbm4.10816","citationCount":"0","resultStr":"{\"title\":\"Trends in Serum Cytokine Expression in Pediatric Skeletal Dysplasia\",\"authors\":\"David A. O'Connell, Ricki S. Carroll, Angela L. Duker, Andrea J. Schelhaas, Marjorie M. Postell, Paul T. Fawcett, Michael B. Bober\",\"doi\":\"10.1002/jbm4.10816\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>The skeletal dysplasias are a heterogeneous group of genetic conditions caused by abnormalities of growth, development, and maintenance of bone and cartilage. Little is known about the roles that cytokines play in the inflammatory and non-inflammatory pathophysiology of skeletal dysplasia. We sought to test our hypothesis that cytokines would be differentially expressed in children with skeletal dysplasia as compared to typically growing controls. Cytokine levels were analyzed using the Cytokine Human Magnetic 25-Plex Panel (Invitrogen, Waltham, MA, USA); 136 growing individuals with skeletal dysplasia and compared to a cohort of 275 healthy pediatric control subjects. We focused on the expression of 12 cytokines across nine dysplasia cohorts. The most common skeletal dysplasia diagnoses were: achondroplasia (58), osteogenesis imperfecta (19), type II collagenopathies (11), multiple epiphyseal dysplasia (MED: 9), diastrophic dysplasia (8), metatropic dysplasia (8), and microcephalic osteodysplastic primordial dwarfism type II (MOPDII: 8). Of the 108 specific observations made, 45 (41.7%) demonstrated statistically significant differences of expression between controls and individuals with skeletal dysplasia. Four of the 12 analyzed cytokines demonstrated elevated expression above control levels in all of the dysplasia cohorts (interleukin 12 [IL-12], IL-13, interferon γ-induced protein 10 kDa [IP-10], regulated on activation, normal T cell expressed and secreted [RANTES]) and two demonstrated expression below control levels across all dysplasia cohorts (monocyte chemoattractant protein 1 [MCP-1], macrophage inflammatory protein-1β [MIP-1β]). The highest levels of overexpression were seen in MOPDII, with expression levels of IP-10 being increased 3.8-fold (<i>p</i> < 0.0001). The lowest statistically significant levels of expressions were in type II collagenopathies, with expression levels of MCP-1 being expressed 0.43-fold lower (<i>p</i> < 0.005). With this data, we hope to lay the groundwork for future directions in dysplasia research that will enhance our understanding of these complex signaling pathways. Looking forward, validating these early trends in cytokine expression, and associating the observed variations with trends in the progression of dysplasia may offer new candidates for clinical biomarkers or even new therapeutics. © 2023 The Authors. <i>JBMR Plus</i> published by Wiley Periodicals LLC. on behalf of American Society for Bone and Mineral Research.</p>\",\"PeriodicalId\":14611,\"journal\":{\"name\":\"JBMR Plus\",\"volume\":\"7 12\",\"pages\":\"\"},\"PeriodicalIF\":3.4000,\"publicationDate\":\"2023-11-09\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://asbmr.onlinelibrary.wiley.com/doi/epdf/10.1002/jbm4.10816\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"JBMR Plus\",\"FirstCategoryId\":\"1085\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/jbm4.10816\",\"RegionNum\":0,\"RegionCategory\":null,\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q2\",\"JCRName\":\"ENDOCRINOLOGY & METABOLISM\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"JBMR Plus","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/jbm4.10816","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q2","JCRName":"ENDOCRINOLOGY & METABOLISM","Score":null,"Total":0}
引用次数: 0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


