用Eschenmoser偶联反应高效合成(Z)-4-((基苯氨基)亚甲基)-异喹啉-1,3(2H,4H)-二酮
IF 2.3
4区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
采用Eschenmoser偶联的新合成策略,将4-溴异喹啉-1,3(2H,4H)-二酮与取代的硫代酰基苯胺、硫代乙酰苯胺和硫代苯并苯胺进行偶联反应,得到18 (Z)-4-(1)。phenylamino -methylidene) isoquinoline-1 3 (2 h, 4 h) -diones。该反应在温和的条件下(DMF或MeCN, 25-60°C)进行,不含任何碱或亲硫剂,产率高(43- 95%)。此外,为了合成携带碱性取代基的起始硫酰亚胺,开发了一种新的硫酰化方案,该方案涉及由CS2和LiBEt3H形成的硫酰化剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
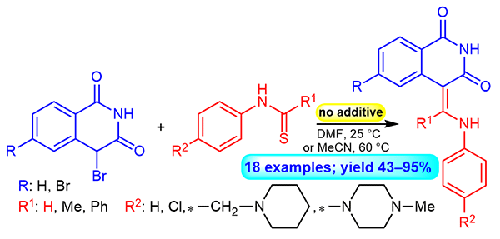
Efficient synthesis of (Z)-4-((subst.phenylamino)methylidene)-isoquinoline-1,3(2H,4H)-diones using Eschenmoser coupling reaction
Novel synthetic strategy involving the Eschenmoser coupling reaction of 4-bromoisoquinoline-1,3(2H,4H)-diones with substituted thioformanilides, thioacetanilides and thiobenzanilides gave 18 (Z)-4-((subst. phenylamino)-methylidene)isoquinoline-1,3(2H,4H)-diones. The reaction occurs under mild conditions (DMF or MeCN, 25-60 °C) without any base or thiophile and in good yields (43-95 %). Furthermore, for the synthesis of starting thioformanilides carrying basic substituents, a new thioacylation protocol was developed that involves a thioacylating agent formed from CS2 and LiBEt3H.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Synthesis-Stuttgart
化学-有机化学
CiteScore
4.50
自引率
7.70%
发文量
435
审稿时长
1 months
期刊介绍:
SYNTHESIS is an international full-paper journal devoted to the advancement of the science of chemical synthesis. It covers all fields of organic chemistry involving synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines. SYNTHESIS provides dependable research results with detailed and reliable experimental procedures and full characterization of all important new products as well as scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


