立体发散diols - alder /Azido-Schmidt反应合成Stemona生物碱(±)-Stenine、(±)-Neostenine和(±)-13- epineostine
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 79
摘要
串联diols - alder /azido-Schmidt反应序列提供了快速进入几种Stemona生物碱共享的核心骨架,包括stenine, neostenine, tuberostemonine和neotuberostemonine。本文描述了这一过程的分子间和分子内变化的发现和演变,以及它们在(±)-stenine和(±)-neostenine全合成中的应用。反应的立体化学结果取决于底物类型和反应条件,使得从相同的二烯/亲二烯组合制备(±)-stenine和(±)-neostenine成为可能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
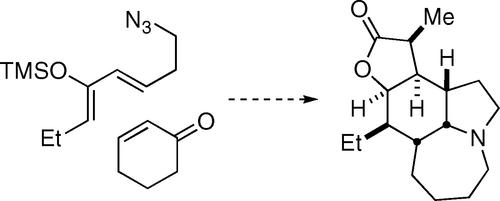
Syntheses of the Stemona Alkaloids (±)-Stenine, (±)-Neostenine, and (±)-13-Epineostenine Using a Stereodivergent Diels–Alder/Azido-Schmidt Reaction
A tandem Diels–Alder/azido-Schmidt reaction sequence provides rapid access to the core skeleton shared by several Stemona alkaloids including stenine, neostenine, tuberostemonine, and neotuberostemonine. The discovery and evolution of inter- and intramolecular variations of this process and their applications to total syntheses of (±)-stenine and (±)-neostenine are described. The stereochemical outcome of the reaction depends on both substrate type and reaction conditions, enabling the preparation of both (±)-stenine and (±)-neostenine from the same diene/dienophile combination.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


