限制热量的抗肿瘤作用由肠道微生物组介导
IF 18.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 10
摘要
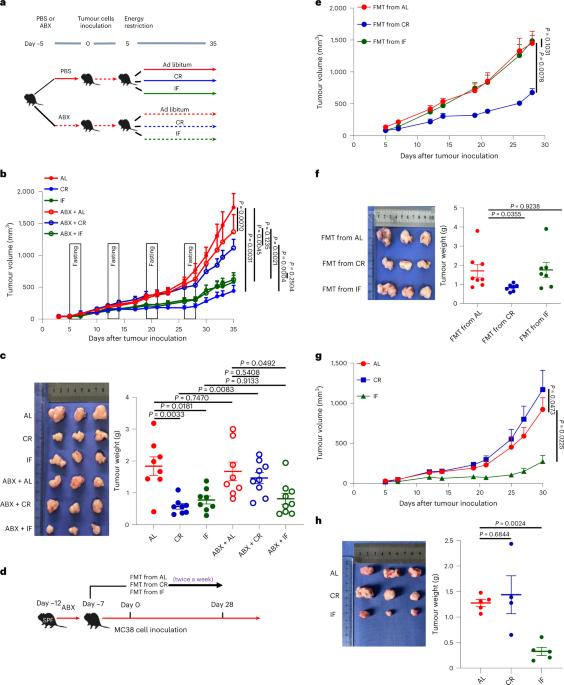
在没有营养不良的情况下限制卡路里摄入量(CR)和间歇性禁食(IF)可降低癌症发病风险。另外,卡路里限制和间歇性禁食还能导致肠道微生物群重塑。然而,肠道微生物群是否在 CR 或 IF 的抗肿瘤效应中发挥作用仍是未知数。在这里,我们研究发现,在雌性小鼠体内,通过依赖肠道微生物群的机制,CR(而非 IF)可防止皮下 MC38 肿瘤的形成。在 CR 之后,我们通过肠道微生物组的 16S rRNA 测序确定了双歧杆菌的富集。此外,在微生物群缺失的小鼠体内施用双歧杆菌足以挽救 CR 的抗肿瘤效果。从机理上讲,双歧杆菌通过产生醋酸盐来介导 CR 诱导的抗肿瘤效应,而这种效应还依赖于肿瘤微环境中干扰素-γ+CD8+ T 细胞的积累。我们的研究结果表明,热量限制可以调节肠道分类组成,这在肿瘤生长动力学和癌症免疫监视方面具有重要的肿瘤学意义。作者的研究表明,热量限制会增加肠道中双歧杆菌的数量,而双歧杆菌又会抑制小鼠的肿瘤发生。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The antitumour effects of caloric restriction are mediated by the gut microbiome
Calorie restriction (CR) and intermittent fasting (IF) without malnutrition reduce the risk of cancer development. Separately, CR and IF can also lead to gut microbiota remodelling. However, whether the gut microbiota has a role in the antitumour effect related to CR or IF is still unknown. Here we show that CR, but not IF, protects against subcutaneous MC38 tumour formation through a mechanism that is dependent on the gut microbiota in female mice. After CR, we identify enrichment of Bifidobacterium through 16S rRNA sequencing of the gut microbiome. Moreover, Bifidobacterium bifidum administration is sufficient to rescue the antitumour effect of CR in microbiota-depleted mice. Mechanistically, B. bifidum mediates the CR-induced antitumour effect through acetate production and this effect is also dependent on the accumulation of interferon-γ+CD8+ T cells in the tumour microenvironment. Our results demonstrate that CR can modulate the gut taxonomic composition, which should be of oncological significance in tumour growth kinetics and cancer immunosurveillance. The authors show that caloric restriction increases the intestinal abundance of Bifidobacterium bifidum, which in turn blunts tumour development in mice.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


