rAAV-PHP.B逃离小鼠眼睛并引起致命性,而rAAV9可以转导无虹膜角膜缘干细胞而没有致命性。
IF 4.6
3区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
最近,人们对高剂量重组腺相关病毒(rAAV)的安全性提出了担忧。因此,我们进行了一系列实验来测试病毒衣壳(rAAV9和rAAV-PHP.B)、剂量和给药途径(基质内、玻璃体内和静脉注射),重点是无虹膜症,这是一种目前无法治愈的先天性失明。无虹膜基因治疗的成功可能取决于受损无虹膜角膜中是否存在功能性角膜缘干细胞(LSCs),以及rAAV是否能转导它们。这两个问题都是未知的,因此我们的研究也解决了这些问题。我们首次报道了玻璃体内或基质内rAAV-PHP后的共济失调和致死率。B病毒注射。我们证明了rAAV9和rAAV-PHP从眼睛逃逸并转导非眼睛组织。B衣壳。我们还表明,rAAV9的基质内和玻璃体内递送可以分别转导无虹膜眼中的功能性LSCs以及所有四种表达PAX6的视网膜细胞类型。总的来说,由于缺乏不良事件以及LSCs和视网膜细胞的成功转导,rAAV9是未来无虹膜基因治疗的首选衣壳。我们对人工注射后rAAV致死性的发现将对开发基于rAAV的基因疗法的其他研究人员产生影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
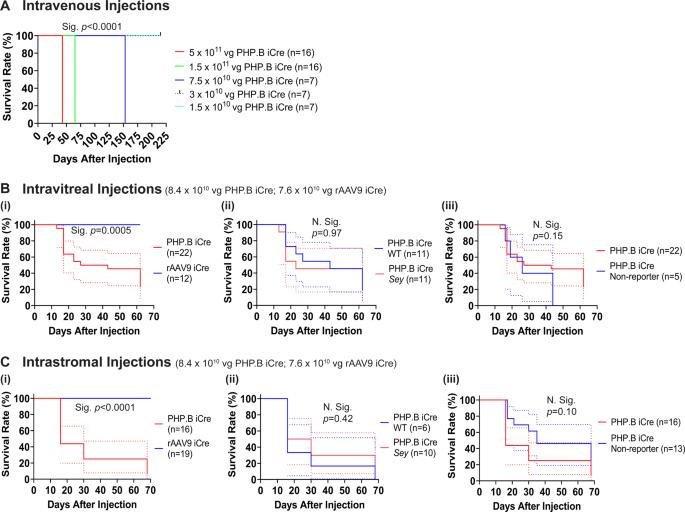
rAAV-PHP.B escapes the mouse eye and causes lethality whereas rAAV9 can transduce aniridic corneal limbal stem cells without lethality
Recently safety concerns have been raised in connection with high doses of recombinant adeno-associated viruses (rAAV). Therefore, we undertook a series of experiments to test viral capsid (rAAV9 and rAAV-PHP.B), dose, and route of administration (intrastromal, intravitreal, and intravenous) focused on aniridia, a congenital blindness that currently has no cure. The success of gene therapy for aniridia may depend on the presence of functional limbal stem cells (LSCs) in the damaged aniridic corneas and whether rAAV can transduce them. Both these concerns were unknown, and thus were also addressed by our studies. For the first time, we report ataxia and lethality after intravitreal or intrastromal rAAV-PHP.B virus injections. We demonstrated virus escape from the eye and transduction of non-ocular tissues by rAAV9 and rAAV-PHP.B capsids. We have also shown that intrastromal and intravitreal delivery of rAAV9 can transduce functional LSCs, as well as all four PAX6-expressing retinal cell types in aniridic eye, respectively. Overall, lack of adverse events and successful transduction of LSCs and retinal cells makes it clear that rAAV9 is the capsid of choice for future aniridia gene therapy. Our finding of rAAV lethality after intraocular injections will be impactful for other researchers developing rAAV-based gene therapies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Gene Therapy
医学-生化与分子生物学
CiteScore
9.70
自引率
2.00%
发文量
67
审稿时长
4-8 weeks
期刊介绍:
Gene Therapy covers both the research and clinical applications of novel therapeutic techniques based on a genetic component. Over the last few decades, significant advances in technologies ranging from identifying novel genetic targets that cause disease through to clinical studies, which show therapeutic benefit, have elevated this multidisciplinary field to the forefront of modern medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


