依鲁替尼用于人体微剂量的碳-13标记
IF 0.9
4区 医学
Q4 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
伊鲁替尼是一种治疗B细胞恶性肿瘤的口服药物。在临床开发过程中,通过静脉微量给药来评估伊鲁替尼的绝对口服生物利用度需要一个稳定的同位素。以下工作描述了从[13C6]苯酚(13C6, 99%)中以31%的总收率生产碳-13标记的依鲁替尼的10步,克级生产,化学纯度为>99%,对映体过量(ee),适用于人体静脉微量给药。本文章由计算机程序翻译,如有差异,请以英文原文为准。
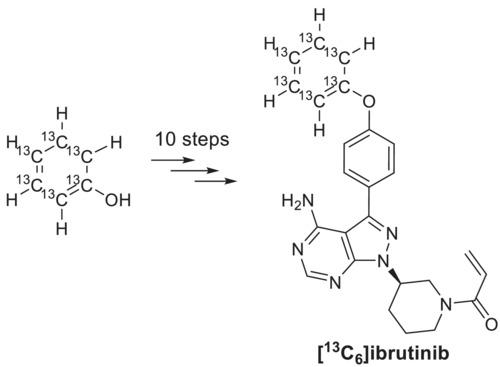
Carbon-13 labeling of ibrutinib for human microdosing
Ibrutinib is an oral medication for the treatment of B cell malignancies. During its clinical development, a stable isotopologue of ibrutinib was required for the assessment of the drug's absolute oral bioavailability via intravenous microdosing. The following work describes a 10-step, gram-scale production of carbon-13 labeled ibrutinib from [13C6]phenol (13C6, 99%) in 31% overall yield with >99% chemical purity and >99% enantiomeric excess (ee), suitable for intravenous microdosing in humans.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.30
自引率
0.00%
发文量
57
审稿时长
1 months
期刊介绍:
The Journal of Labelled Compounds and Radiopharmaceuticals publishes all aspects of research dealing with labeled compound preparation and applications of these compounds. This includes tracer methods used in medical, pharmacological, biological, biochemical and chemical research in vitro and in vivo.
The Journal of Labelled Compounds and Radiopharmaceuticals devotes particular attention to biomedical research, diagnostic and therapeutic applications of radiopharmaceuticals, covering all stages of development from basic metabolic research and technological development to preclinical and clinical studies based on physically and chemically well characterized molecular structures, coordination compounds and nano-particles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


