Norah A Owiti, Simran Kaushal, Lincoln Martin, Jamie Sly, Carol D Swartz, Jasmine Fowler, Joshua J Corrigan, Les Recio, Bevin P Engelward
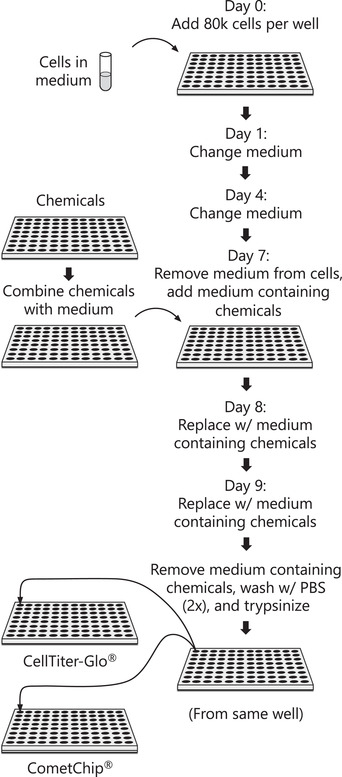
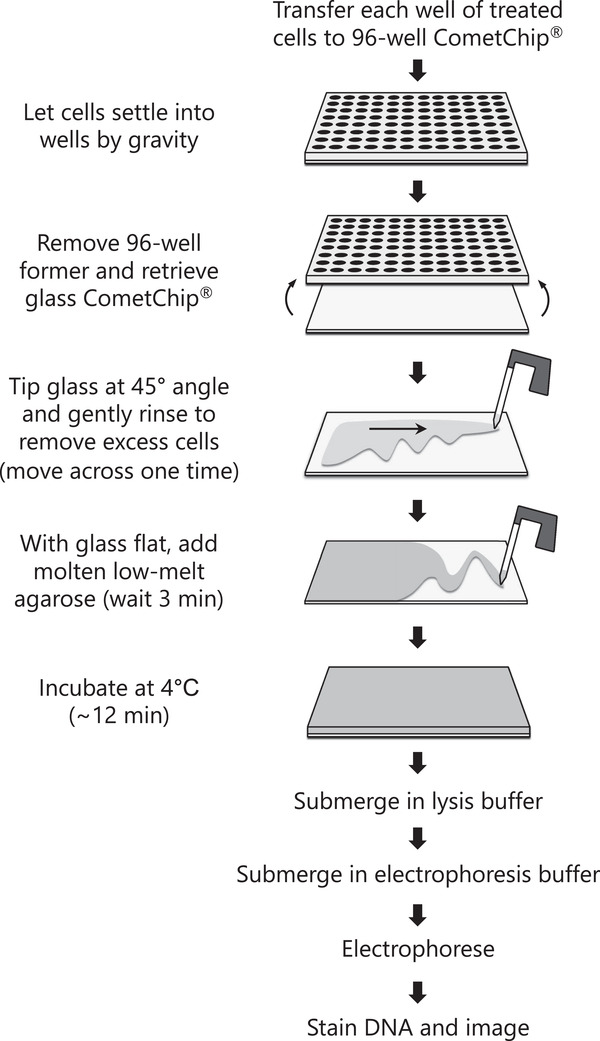
{"title":"使用HepaCometChip法进行广谱DNA损伤分析。","authors":"Norah A Owiti, Simran Kaushal, Lincoln Martin, Jamie Sly, Carol D Swartz, Jasmine Fowler, Joshua J Corrigan, Les Recio, Bevin P Engelward","doi":"10.1002/cpz1.563","DOIUrl":null,"url":null,"abstract":"<p><p>Exposure to DNA damaging agents can lead to mutations that cause cancer. The liver is particularly vulnerable because it contains high levels of Cytochrome P450 enzymes that can convert xenobiotics into DNA reactive metabolites that form potentially carcinogenic bulky DNA adducts. As such, current requirements for preclinical testing include in vivo testing for DNA damage in the liver, which often requires many animals. Given that efforts are underway in many countries to reduce or eliminate the use of animals in research, there is a critical need for fast and robust in vitro tests to discern whether xenobiotics or potential pharmaceutical agents can damage the hepatocyte genome. One possible approach is to leverage the alkaline comet assay, which is used to assess genotoxicity based on the ability of damaged DNA to become free to migrate toward the anode during electrophoresis. The comet assay, however, has several limitations. The assay is (i) slow and (ii) vulnerable to experimental noise, (iii) it is difficult to detect bulky DNA adducts since they do not directly affect DNA migration, and (iv) cell types typically used do not have robust metabolic capacity. To address some of these concerns, we have developed the \"HepaCometChip\" (a.k.a. the HepaRG CometChip), wherein metabolically competent cells are incorporated into a higher throughput CometChip platform. Repair trapping is used to increase sensitivity for bulky lesions: undetectable bulky lesions are converted into repair intermediates (specifically, single-strand breaks) that can be detected with the assay. Here, we describe a protocol for performing the HepaCometChip assay that includes handling and dosing of HepaRG cells and performing the CometChip assay. With its higher throughput, ability to capture metabolic activation, and sensitivity to bulky lesions, the HepaCometChip offers a potential alternative to the use of animals for genotoxicity testing. © 2022 The Authors. Current Protocols published by Wiley Periodicals LLC. Basic Protocol 1: HepaRG cell culturing and dosing Basic Protocol 2: CometChip assay.</p>","PeriodicalId":11174,"journal":{"name":"Current Protocols","volume":"2 9","pages":"e563"},"PeriodicalIF":0.0000,"publicationDate":"2022-09-01","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://ftp.ncbi.nlm.nih.gov/pub/pmc/oa_pdf/1e/5d/CPZ1-2-0.PMC9522315.pdf","citationCount":"0","resultStr":"{\"title\":\"Using the HepaCometChip Assay for Broad-Spectrum DNA Damage Analysis.\",\"authors\":\"Norah A Owiti, Simran Kaushal, Lincoln Martin, Jamie Sly, Carol D Swartz, Jasmine Fowler, Joshua J Corrigan, Les Recio, Bevin P Engelward\",\"doi\":\"10.1002/cpz1.563\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p><p>Exposure to DNA damaging agents can lead to mutations that cause cancer. The liver is particularly vulnerable because it contains high levels of Cytochrome P450 enzymes that can convert xenobiotics into DNA reactive metabolites that form potentially carcinogenic bulky DNA adducts. As such, current requirements for preclinical testing include in vivo testing for DNA damage in the liver, which often requires many animals. Given that efforts are underway in many countries to reduce or eliminate the use of animals in research, there is a critical need for fast and robust in vitro tests to discern whether xenobiotics or potential pharmaceutical agents can damage the hepatocyte genome. One possible approach is to leverage the alkaline comet assay, which is used to assess genotoxicity based on the ability of damaged DNA to become free to migrate toward the anode during electrophoresis. The comet assay, however, has several limitations. The assay is (i) slow and (ii) vulnerable to experimental noise, (iii) it is difficult to detect bulky DNA adducts since they do not directly affect DNA migration, and (iv) cell types typically used do not have robust metabolic capacity. To address some of these concerns, we have developed the \\\"HepaCometChip\\\" (a.k.a. the HepaRG CometChip), wherein metabolically competent cells are incorporated into a higher throughput CometChip platform. Repair trapping is used to increase sensitivity for bulky lesions: undetectable bulky lesions are converted into repair intermediates (specifically, single-strand breaks) that can be detected with the assay. Here, we describe a protocol for performing the HepaCometChip assay that includes handling and dosing of HepaRG cells and performing the CometChip assay. With its higher throughput, ability to capture metabolic activation, and sensitivity to bulky lesions, the HepaCometChip offers a potential alternative to the use of animals for genotoxicity testing. © 2022 The Authors. Current Protocols published by Wiley Periodicals LLC. Basic Protocol 1: HepaRG cell culturing and dosing Basic Protocol 2: CometChip assay.</p>\",\"PeriodicalId\":11174,\"journal\":{\"name\":\"Current Protocols\",\"volume\":\"2 9\",\"pages\":\"e563\"},\"PeriodicalIF\":0.0000,\"publicationDate\":\"2022-09-01\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://ftp.ncbi.nlm.nih.gov/pub/pmc/oa_pdf/1e/5d/CPZ1-2-0.PMC9522315.pdf\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"Current Protocols\",\"FirstCategoryId\":\"1085\",\"ListUrlMain\":\"https://doi.org/10.1002/cpz1.563\",\"RegionNum\":0,\"RegionCategory\":null,\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"\",\"JCRName\":\"\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current Protocols","FirstCategoryId":"1085","ListUrlMain":"https://doi.org/10.1002/cpz1.563","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"","JCRName":"","Score":null,"Total":0}
引用次数: 0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


