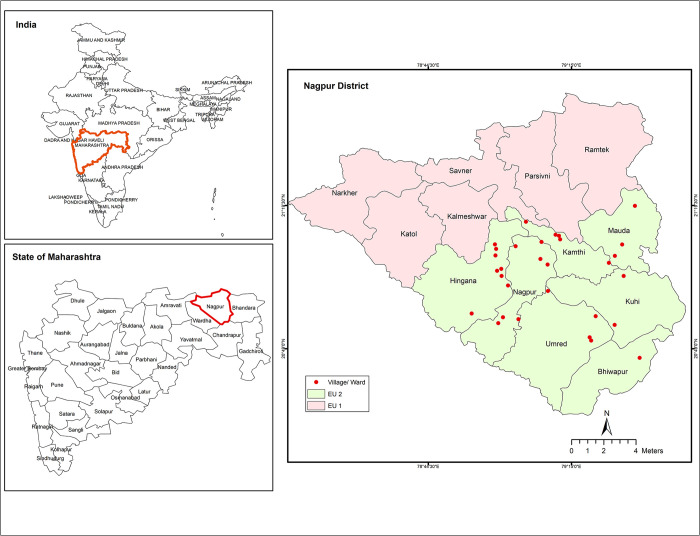
在印度马哈拉施特拉邦那格浦尔区的一个评估单位,用三种药物方案进行大规模药物管理的覆盖率评估。
IF 3.4
2区 医学
Q1 Medicine
PLoS Neglected Tropical Diseases
Pub Date : 2023-09-07
eCollection Date: 2023-09-01
DOI:10.1371/journal.pntd.0011588
引用次数: 0
摘要
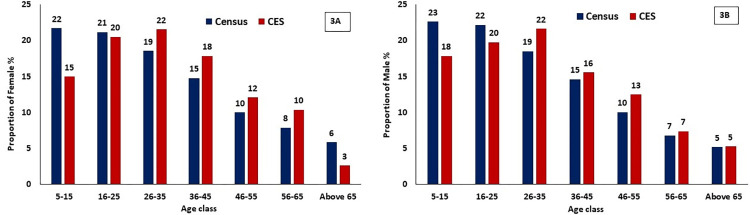
背景:2018年12月,印度启动了加速消除淋巴丝虫病的三重药物方案(IDA;伊维菌素、二乙基氨基甲嗪、阿苯达唑)。马哈拉施特拉邦的那格浦尔区是首批引入这一战略的五个区之一。该地区的国家病媒传播疾病控制计划(NVBDCP)报告称,在2019年1月那格浦尔地区EU-2实施的IDA第一轮大规模药物管理(MDA)中,治疗覆盖率约为85.0%。根据国家指南,进行了覆盖率评估调查,收集了定量和定性数据,以评估治疗覆盖率、社区准备水平,并确定需要改进的差距(如果有的话)。方法:根据世界卫生组织建议的方案,于2019年3月在那格浦尔区两个评估单位之一(EU-2)进行了覆盖率评估调查(CES)。覆盖率样本生成器(CSB)V2.9工具用于计算样本量、选择位点和估计药物覆盖率。CSB工具遵循两阶段整群抽样程序,选择30个主要抽样单位(作为一个整群的病房/村庄)和一份随机数列表,用于在每个整群中选择家庭。对结果进行了操作指标分析。Stata第14.0版软件用于构建95%的置信限,以解释聚类。结果:从EU-2中随机选择的30个集群中,共调查了来自328个HH的1601名5-85岁的男女个体。平均年龄33.8±17.6岁。在接受调查的人群中,78.0%的人接受了药物(计划覆盖范围),66.1%的人服用了药物(调查覆盖范围)。农村地区(82.6%)的调查覆盖率显著高于城市地区(59.4%)和城市周边地区(58.6%)(P结论:在那格浦尔区的欧盟2区,基于IDA的MDA策略可以达到所需的治疗覆盖率水平(~65%),该区此前已进行了几轮DA MDA(二乙基氨基甲嗪、阿苯达唑)。在农村地区实现了>80%的有效治疗覆盖率后,需要提高城市和城市周边地区的覆盖率,以达到IDA-MDA的影响。必须加强药物交付和社区准备活动,同时改善DOT,特别是在城市和城郊地区,以达到所需的治疗覆盖水平。添加伊维菌素没有任何额外的不良事件。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Coverage evaluation of mass drug administration with triple drug regimen in an evaluation unit in Nagpur district of Maharashtra, India.
Background Triple drug regimen (IDA; Ivermectin, Diethylcarbamazine, Albendazole) recommended for accelerating elimination of lymphatic filariasis was launched in India in December 2018. Nagpur district in Maharashtra was one of the first five districts where this strategy was introduced. The National Vector Borne Disease Control Programme (NVBDCP) at the district reported ~85.0% treatment coverage in the first round of mass drug administration (MDA) with IDA implemented in EU-2 in Nagpur district in January 2019. As per the national guideline, a coverage evaluation survey was carried out and both quantitative and qualitative data were collected to assess the treatment coverage, the level of community preparation and identify the gaps, if any, for improvement. Methodology A Coverage Evaluation Survey (CES) following the WHO recommended protocol was conducted in one of the two evaluation units (EU-2) in Nagpur district in March 2019. Coverage Sample Builder (CSB) V2.9 tool was used to calculate the sample size, select sites and estimate drug coverage. The CSB tool followed a two-stage cluster sampling procedure to select 30 primary sampling units (ward/village as a cluster) and a list of random numbers for selecting households (HHs) in each cluster. The results were analyzed for operational indicators. Stata ver. 14.0 software was used to construct the 95% confidence limits accounting for clustering. Results A total of 1601 individuals aged 5–85 years of both gender from 328 HHs were surveyed from the 30 randomly selected clusters in EU-2. The mean age was 33.8±17.6 years. Among the surveyed population, 78.0% received the drugs (programme reach) and 66.1% consumed the drugs (survey coverage). Survey coverage was significantly higher in rural (82.6%) than in urban (59.4%) and peri-urban (58.6%) areas (P<0.001). Directly observed treatment (DOT) among the surveyed population was 51.6%. Adverse events were reported among 6.9% respondents who reported to have consumed the drugs. Conclusion The IDA based MDA strategy could achieve just the required level of treatment coverage (~65%) in EU-2, Nagpur district, which had previously undergone several rounds of DA-MDAs (Diethylcarbamazine, Albendazole). Having achieved an effective treatment coverage of >80% in rural areas, the coverage in urban and peri-urban areas need to be improved in order to attain the impact of IDA-MDA. It is imperative to strengthen drug delivery and community preparation activities along with improved DOT especially in urban and peri-urban areas to achieve the required level of treatment coverage. Addition of ivermectin did not have any additional perceived adverse events.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

PLoS Neglected Tropical Diseases
Medicine-Infectious Diseases
CiteScore
7.40
自引率
10.50%
发文量
723
审稿时长
2-3 weeks
期刊介绍:
PLOS Neglected Tropical Diseases publishes research devoted to the pathology, epidemiology, prevention, treatment and control of the neglected tropical diseases (NTDs), as well as relevant public policy.
The NTDs are defined as a group of poverty-promoting chronic infectious diseases, which primarily occur in rural areas and poor urban areas of low-income and middle-income countries. Their impact on child health and development, pregnancy, and worker productivity, as well as their stigmatizing features limit economic stability.
All aspects of these diseases are considered, including:
Pathogenesis
Clinical features
Pharmacology and treatment
Diagnosis
Epidemiology
Vector biology
Vaccinology and prevention
Demographic, ecological and social determinants
Public health and policy aspects (including cost-effectiveness analyses).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


