利用仿生铁卟啉催化氧化高效合成杀菌剂星比脲的碳14标记代谢物
IF 0.9
4区 医学
Q4 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
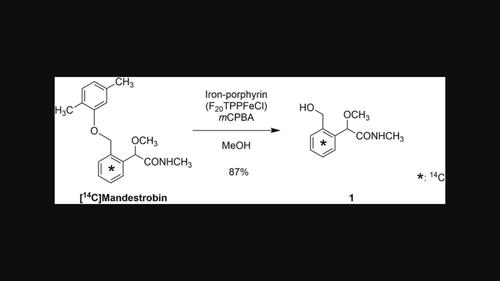
以合成铁卟啉(F20TPPFeCl)为催化剂进行仿生氧化,去除杀菌剂manderbin的二甲苯部分,在苯环上均匀标记碳-14,生成相应的放射性标记代谢物1。通过对中间体5和副产物对二甲苯醌衍生物6、7的化学结构进行鉴定,研究了反应机理。在此基础上,对反应因素进行优化,使山竹碱的产率提高到87%。最后,从1中制备了各种碳-14标记的manderisbin代谢物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Efficient synthesis of carbon-14 labeled metabolites of the strobilurin fungicide mandestrobin using biomimetic iron-porphyrin catalyzed oxidation
Biomimetic oxidation using synthetic iron-porphyrin (F20 TPPFeCl) as a catalyst eliminated a xylene moiety of the fungicide mandestrobin, uniformly labeled with carbon-14 at the benzyl ring, to produce the corresponding radiolabeled metabolite 1. This reaction mechanism was investigated by identifying chemical structures of intermediate 5 and p-xyloquinone derivatives 6 and 7, as by-products. Optimization of reaction factors based on the mechanism improved the yield of 1 from mandestrobin up to 87%. Finally, various carbon-14 labeled metabolites of mandestrobin were prepared from 1.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.30
自引率
0.00%
发文量
57
审稿时长
1 months
期刊介绍:
The Journal of Labelled Compounds and Radiopharmaceuticals publishes all aspects of research dealing with labeled compound preparation and applications of these compounds. This includes tracer methods used in medical, pharmacological, biological, biochemical and chemical research in vitro and in vivo.
The Journal of Labelled Compounds and Radiopharmaceuticals devotes particular attention to biomedical research, diagnostic and therapeutic applications of radiopharmaceuticals, covering all stages of development from basic metabolic research and technological development to preclinical and clinical studies based on physically and chemically well characterized molecular structures, coordination compounds and nano-particles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


