应激引起的胃功能障碍的大脑调节。
IF 20.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
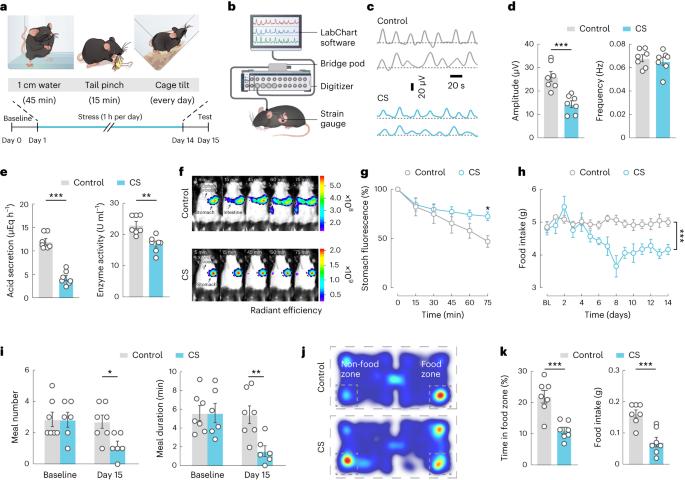
心理和身体压力源与人类的胃疾病有关。应激诱导的胃功能障碍背后的大脑与胃的耦合机制仍然难以捉摸。在这里,我们发现胃直接接收来自迷走神经背侧运动核(AChDMV)的乙酰胆碱能输入,迷走神经由中缝背侧核(5-HTDRN)中的5-羟色胺能神经元支配。显微钙成像和多四极电生理记录显示5-HTDRN → AChDMV → 慢性应激伴随胃功能低下,胃回路受到抑制。该回路的人工激活可逆转雄性和雌性小鼠由慢性应激诱导的胃功能障碍。我们的研究表明,这种5-HTDRN → AChDMV → 胃轴驱动与压力相关的胃功能障碍,从而为大脑调节胃提供了深入的电路基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Brain regulation of gastric dysfunction induced by stress
Psychological and physical stressors have been implicated in gastric disorders in humans. The mechanism coupling the brain to the stomach underlying stress-induced gastric dysfunction has remained elusive. Here, we show that the stomach directly receives acetylcholinergic inputs from the dorsal motor nucleus of the vagus (AChDMV), which are innervated by serotonergic neurons in the dorsal raphe nucleus (5-HTDRN). Microendoscopic calcium imaging and multi-tetrode electrophysiological recordings reveal that the 5-HTDRN → AChDMV → stomach circuit is inhibited with chronic stress accompanied by hypoactivate gastric function. Artificial activation of this circuit reverses the gastric dysfunction induced by chronic stress in both male and female mice. Our study demonstrates that this 5-HTDRN → AChDMV → stomach axis drives gastric dysfunction associated with stress, thus providing insights into the circuit basis for brain regulation of the stomach. The authors uncover a brain-to-stomach signalling axis that communicates gastric dysfunction associated with stress.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


