小鼠玻璃体内给药后合理设计的AAV2衣壳的免疫生物学。
IF 4.6
3区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
腺相关病毒血清型2(AAV2)是一种病毒载体,可用于向视网膜中的病变细胞递送治疗基因。改变AAV2载体的一种策略涉及磷酸降解子残基的突变,磷酸降解子被认为在胞质溶胶中被磷酸化/泛素化,促进载体的降解和转导的抑制。因此,磷降解蛋白残基的突变与靶细胞转导的增加有关,然而,目前的文献中缺乏对玻璃体内(IVT)递送至免疫活性动物后野生型和磷降解蛋白突变AAV2载体的免疫生物学的评估。在这项研究中,我们发现,与野生型AAV2衣壳相比,三磷酸降解蛋白突变体AAV2衣衣壳的IVT与更高水平的体液免疫激活、CD4和CD8 T细胞向视网膜的浸润、脾脏生发中心反应的产生、传统树突状细胞亚群的激活以及视网膜胶质增生升高有关。然而,我们没有检测到载体给药后视网膜电图的显著变化。我们还证明,三重AAV2突变体衣壳不太容易被可溶性硫酸乙酰肝素和抗AAV2中和抗体中和,这突出了该载体在规避先前存在的体液免疫方面的可能效用。总之,本研究强调了合理设计的载体免疫生物学的新方面,这可能与它们在临床前和临床环境中的应用有关。本文章由计算机程序翻译,如有差异,请以英文原文为准。
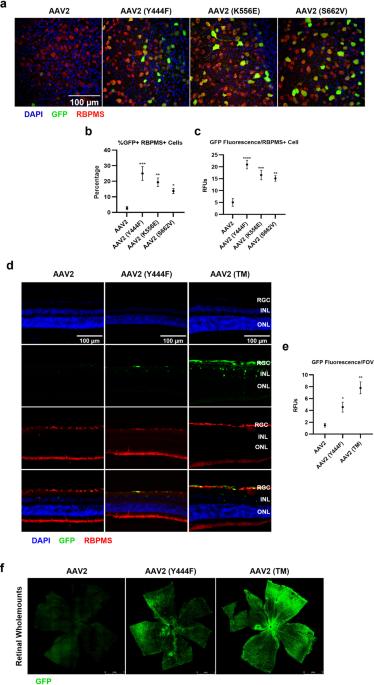
Immunobiology of a rationally-designed AAV2 capsid following intravitreal delivery in mice
Adeno-associated virus serotype 2 (AAV2) is a viral vector that can be used to deliver therapeutic genes to diseased cells in the retina. One strategy for altering AAV2 vectors involves the mutation of phosphodegron residues, which are thought to be phosphorylated/ubiquitinated in the cytosol, facilitating degradation of the vector and the inhibition of transduction. As such, mutation of phosphodegron residues have been correlated with increased transduction of target cells, however, an assessment of the immunobiology of wild-type and phosphodegron mutant AAV2 vectors following intravitreal (IVT) delivery to immunocompetent animals is lacking in the current literature. In this study, we show that IVT of a triple phosphodegron mutant AAV2 capsid is associated with higher levels of humoral immune activation, infiltration of CD4 and CD8 T-cells into the retina, generation of splenic germinal centre reactions, activation of conventional dendritic cell subsets, and elevated retinal gliosis compared to wild-type AAV2 capsids. However, we did not detect significant changes in electroretinography arising after vector administration. We also demonstrate that the triple AAV2 mutant capsid is less susceptible to neutralisation by soluble heparan sulphate and anti-AAV2 neutralising antibodies, highlighting a possible utility for the vector in terms of circumventing pre-existing humoral immunity. In summary, the present study highlights novel aspects of rationally-designed vector immunobiology, which may be relevant to their application in preclinical and clinical settings.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Gene Therapy
医学-生化与分子生物学
CiteScore
9.70
自引率
2.00%
发文量
67
审稿时长
4-8 weeks
期刊介绍:
Gene Therapy covers both the research and clinical applications of novel therapeutic techniques based on a genetic component. Over the last few decades, significant advances in technologies ranging from identifying novel genetic targets that cause disease through to clinical studies, which show therapeutic benefit, have elevated this multidisciplinary field to the forefront of modern medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


