Marta Persson, Mattias K Andersson, Per-Erik Sahlin, Yoshitsugu Mitani, Margaret S Brandwein-Weber, Henry F Frierson Jr, Christopher Moskaluk, Isabel Fonseca, Renata Ferrarotto, Werner Boecker, Thomas Loening, Adel K El-Naggar, Göran Stenman
下载PDF
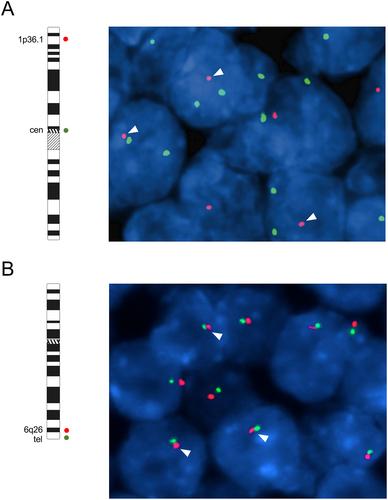
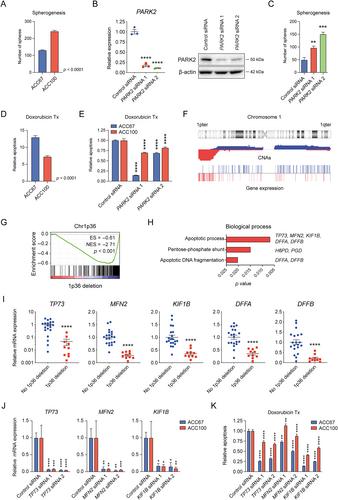
{"title":"腺样囊性癌的综合分子特征揭示肿瘤抑制剂是新的驱动因素和预后生物标志物","authors":"Marta Persson, Mattias K Andersson, Per-Erik Sahlin, Yoshitsugu Mitani, Margaret S Brandwein-Weber, Henry F Frierson Jr, Christopher Moskaluk, Isabel Fonseca, Renata Ferrarotto, Werner Boecker, Thomas Loening, Adel K El-Naggar, Göran Stenman","doi":"10.1002/path.6172","DOIUrl":null,"url":null,"abstract":"<p>Adenoid cystic carcinoma (ACC) is a MYB-driven head and neck malignancy with high rates of local recurrence and distant metastasis and poor long-term survival. New effective targeted therapies and clinically useful biomarkers for patient stratification are needed to improve ACC patient survival. Here, we present an integrated copy number and transcriptomic analysis of ACC to identify novel driver genes and prognostic biomarkers. A total of 598 ACCs were studied. Clinical follow-up was available from 366 patients, the largest cohort analyzed to date. Copy number losses of 1p36 (70/492; 14%) and of the tumor suppressor gene <i>PARK2</i> (6q26) (85/343; 25%) were prognostic biomarkers; patients with concurrent losses (<i>n</i> = 20) had significantly shorter overall survival (OS) than those with one or no deletions (<i>p</i> < 0.0001). Deletion of 1p36 independently predicted short OS in multivariate analysis (<i>p</i> = 0.02). Two pro-apoptotic genes, <i>TP73</i> and <i>KIF1B</i>, were identified as putative 1p36 tumor suppressor genes whose reduced expression was associated with poor survival and increased resistance to apoptosis. <i>PARK2</i> expression was markedly reduced in tumors with 6q deletions, and <i>PARK2</i> knockdown increased spherogenesis and decreased apoptosis, indicating that <i>PARK2</i> is a tumor suppressor in ACC. Moreover, analysis of the global gene expression pattern in 30 ACCs revealed a transcriptomic signature associated with short OS, multiple copy number alterations including 1p36 deletions, and reduced expression of <i>TP73</i>. Taken together, the results indicate that <i>TP73</i> and <i>PARK2</i> are novel putative tumor suppressor genes and potential prognostic biomarkers in ACC. Our studies provide new important insights into the pathogenesis of ACC. The results have important implications for biomarker-driven stratification of patients in clinical trials. © 2023 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"261 3","pages":"256-268"},"PeriodicalIF":5.6000,"publicationDate":"2023-08-11","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6172","citationCount":"0","resultStr":"{\"title\":\"Comprehensive molecular characterization of adenoid cystic carcinoma reveals tumor suppressors as novel drivers and prognostic biomarkers\",\"authors\":\"Marta Persson, Mattias K Andersson, Per-Erik Sahlin, Yoshitsugu Mitani, Margaret S Brandwein-Weber, Henry F Frierson Jr, Christopher Moskaluk, Isabel Fonseca, Renata Ferrarotto, Werner Boecker, Thomas Loening, Adel K El-Naggar, Göran Stenman\",\"doi\":\"10.1002/path.6172\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>Adenoid cystic carcinoma (ACC) is a MYB-driven head and neck malignancy with high rates of local recurrence and distant metastasis and poor long-term survival. New effective targeted therapies and clinically useful biomarkers for patient stratification are needed to improve ACC patient survival. Here, we present an integrated copy number and transcriptomic analysis of ACC to identify novel driver genes and prognostic biomarkers. A total of 598 ACCs were studied. Clinical follow-up was available from 366 patients, the largest cohort analyzed to date. Copy number losses of 1p36 (70/492; 14%) and of the tumor suppressor gene <i>PARK2</i> (6q26) (85/343; 25%) were prognostic biomarkers; patients with concurrent losses (<i>n</i> = 20) had significantly shorter overall survival (OS) than those with one or no deletions (<i>p</i> < 0.0001). Deletion of 1p36 independently predicted short OS in multivariate analysis (<i>p</i> = 0.02). Two pro-apoptotic genes, <i>TP73</i> and <i>KIF1B</i>, were identified as putative 1p36 tumor suppressor genes whose reduced expression was associated with poor survival and increased resistance to apoptosis. <i>PARK2</i> expression was markedly reduced in tumors with 6q deletions, and <i>PARK2</i> knockdown increased spherogenesis and decreased apoptosis, indicating that <i>PARK2</i> is a tumor suppressor in ACC. Moreover, analysis of the global gene expression pattern in 30 ACCs revealed a transcriptomic signature associated with short OS, multiple copy number alterations including 1p36 deletions, and reduced expression of <i>TP73</i>. Taken together, the results indicate that <i>TP73</i> and <i>PARK2</i> are novel putative tumor suppressor genes and potential prognostic biomarkers in ACC. Our studies provide new important insights into the pathogenesis of ACC. The results have important implications for biomarker-driven stratification of patients in clinical trials. © 2023 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>\",\"PeriodicalId\":232,\"journal\":{\"name\":\"The Journal of Pathology\",\"volume\":\"261 3\",\"pages\":\"256-268\"},\"PeriodicalIF\":5.6000,\"publicationDate\":\"2023-08-11\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6172\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"The Journal of Pathology\",\"FirstCategoryId\":\"3\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/path.6172\",\"RegionNum\":2,\"RegionCategory\":\"医学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q1\",\"JCRName\":\"ONCOLOGY\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6172","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


