一种新的质子激活氯化物通道的多模式门控模型。
IF 7.8
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
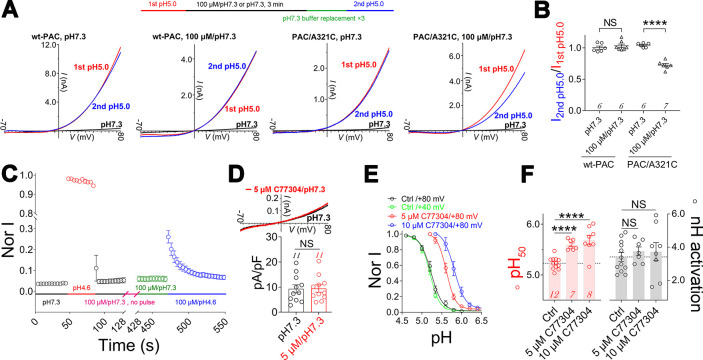
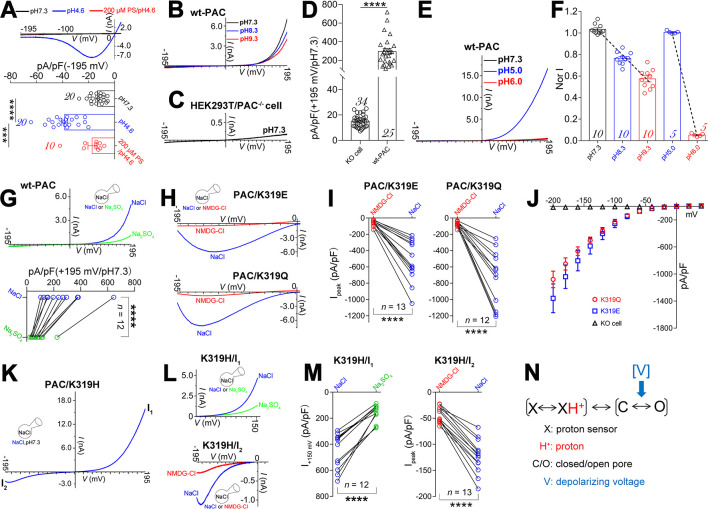
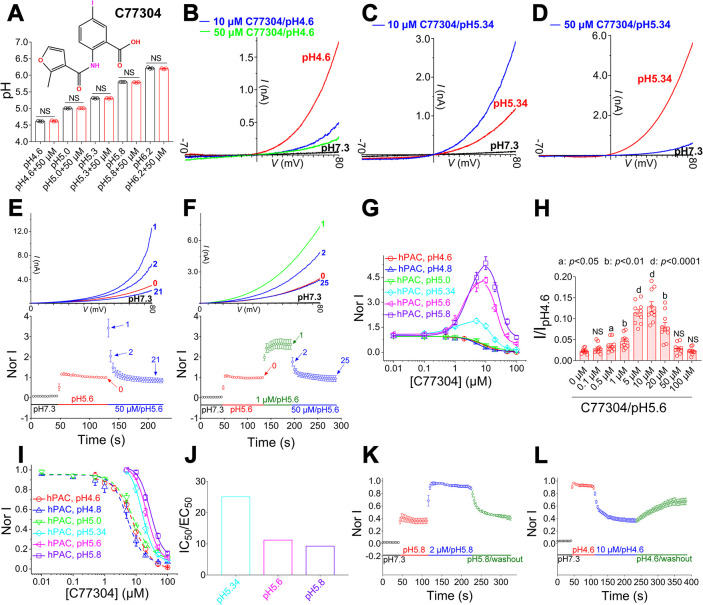
质子激活的氯化物(PAC)通道在缺血性神经元死亡中起着关键作用,但其激活机制尚不明确。在此,我们使用其新型双功能调制器C77304研究了PAC通道的门控。C77304作为PAC通道的弱激活剂,通过作用于其质子门控而引起适度激活。然而,在较高浓度下,C77304作为弱抑制剂,抑制通道活性。这种双重功能是通过与通道的2个调节位点相互作用来实现的,每个调节位点对通道的状态具有不同的亲和力和依赖性。此外,我们发现PAC通道的质子化无关电压激活似乎通过离子通量门控机制运行。通过扫描诱变和分子动力学模拟,我们证实人类PAC通道中的E181、E257和E261是主要的质子传感器,因为它们的丙氨酸突变消除了通道的质子门控,同时保留了电压依赖性门控。这种质子传感机制在来自不同物种的同源PAC通道中是保守的。总之,我们的数据揭示了PAC通道中的多模式门控和质子传感机制,这可能会激发潜在的药物开发。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A new polymodal gating model of the proton-activated chloride channel.
The proton-activated chloride (PAC) channel plays critical roles in ischemic neuron death, but its activation mechanisms remain elusive. Here, we interrogated PAC channel gating using its unique bidirectional modulator C77304 as a pharmacological probe. C77304 activated the PAC channel by acting on its proton gating, while simultaneously inhibiting channel activity at higher doses, through interaction with two modulatory sites with different affinities and state-dependence. Excitingly, we revealed that PAC undergoes intrinsic proton gating-independent voltage activation, which was defined by an ion-flux gating mechanism. Scanning-mutagenesis and molecular dynamics simulation confirmed that E181, E257, and E261 in human PAC form the primary proton sensors, as alanine mutations eliminated the channel’s proton gating while sparing the voltage-dependent gating. This proton sensing mechanism was basically conserved among orthologous PAC channels. Collectively, our data unveils the polymodal gating and proton sensing mechanisms in the PAC channel which may inspire potential drug development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

PLoS Biology
生物-生化与分子生物学
CiteScore
14.40
自引率
2.00%
发文量
359
审稿时长
3 months
期刊介绍:
PLOS Biology is an open-access, peer-reviewed general biology journal published by PLOS, a nonprofit organization of scientists and physicians dedicated to making the world's scientific and medical literature freely accessible. The journal publishes new articles online weekly, with issues compiled and published monthly.
ISSN Numbers:
eISSN: 1545-7885
ISSN: 1544-9173
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


