Srsf2P95H/+ 与 TET2 缺失共同作用,促进髓系偏向,并引发小鼠慢性粒细胞白血病样疾病
IF 13.4
1区 医学
Q1 HEMATOLOGY
引用次数: 2
摘要
RNA 剪接蛋白和表观遗传调节因子的复发性突变是导致骨髓增生异常综合征(MDS)和相关髓系肿瘤发生的原因之一。在慢性粒细胞白血病(CMML)中,约50%的患者发生SRSF2突变,约60%的患者发生TET2突变。克隆分析表明,任何一种突变都可能作为创始病变出现。基于人类癌症遗传学,我们将可诱导的Srsf2P95H/+突变模型与Tet2fl/fl小鼠杂交,使两者在造血干细胞中同时发生突变。诱导突变后20-24周,我们观察到Srsf2/Tet2突变体与单一突变体相比有细微差别。在衰老的原生造血条件下,我们发现Srsf2/Tet2突变体有明显的骨髓偏向和单核细胞增多现象。Srsf2/Tet2复合突变体的一个子集显示粒细胞和独特的单核细胞增生(髓单核细胞增生)增加,未成熟原核细胞、单核细胞和双核原核细胞增加。对进展期疾病的外显子组分析表明,其基因和通路的突变与人类 CMML 中报告的基因和通路的突变相似。移植后,受者出现白细胞增多、单核细胞增多和脾脏肿大。我们在体内重现了Srsf2/Tet2的协同作用,产生了一种具有CMML核心特征的疾病,与单一的Srsf2或Tet2突变不同。该模型是朝着建立高保真和遗传上可控的 CMML 模型迈出的重要一步。本文章由计算机程序翻译,如有差异,请以英文原文为准。
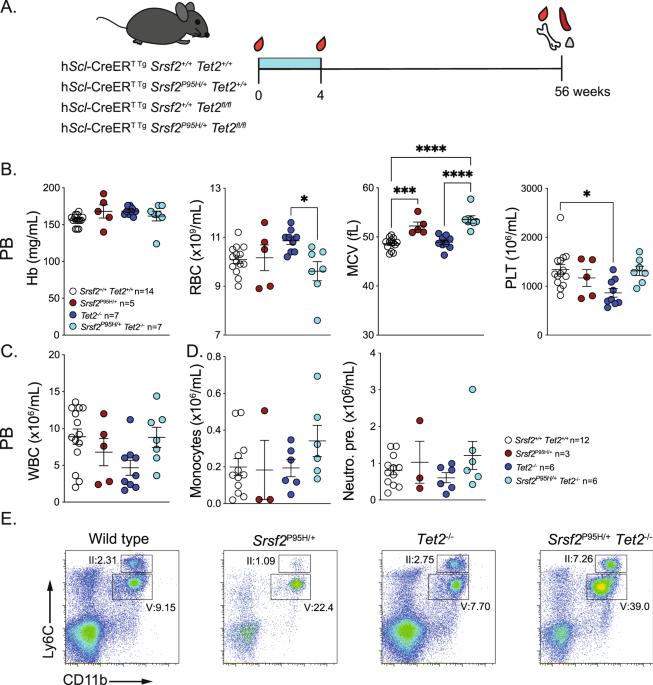
Srsf2P95H/+ co-operates with loss of TET2 to promote myeloid bias and initiate a chronic myelomonocytic leukemia-like disease in mice
Recurrent mutations in RNA splicing proteins and epigenetic regulators contribute to the development of myelodysplastic syndrome (MDS) and related myeloid neoplasms. In chronic myelomonocytic leukemia (CMML), SRSF2 mutations occur in ~50% of patients and TET2 mutations in ~60%. Clonal analysis indicates that either mutation can arise as the founder lesion. Based on human cancer genetics we crossed an inducible Srsf2P95H/+ mutant model with Tet2fl/fl mice to mutate both concomitantly in hematopoietic stem cells. At 20–24 weeks post mutation induction, we observed subtle differences in the Srsf2/Tet2 mutants compared to either single mutant. Under conditions of native hematopoiesis with aging, we see a distinct myeloid bias and monocytosis in the Srsf2/Tet2 mutants. A subset of the compound Srsf2/Tet2 mutants display an increased granulocytic and distinctive monocytic proliferation (myelomonocytic hyperplasia), with increased immature promonocytes and monoblasts and binucleate promonocytes. Exome analysis of progressed disease demonstrated mutations in genes and pathways similar to those reported in human CMML. Upon transplantation, recipients developed leukocytosis, monocytosis, and splenomegaly. We reproduce Srsf2/Tet2 co-operativity in vivo, yielding a disease with core characteristics of CMML, unlike single Srsf2 or Tet2 mutation. This model represents a significant step toward building high fidelity and genetically tractable models of CMML.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


