PD-L2 - RGMb信号缺失引起的微生物群相关免疫治疗耐药。
IF 7.2
2区 医学
引用次数: 0
摘要
Park等人在《Nature》杂志最近发表的一篇论文中提出了肠道生态失调影响针对PD-L1/PD-1相互作用的免疫治疗效果的机制。生态失调可能上调一对检查点分子,即PD-L2与RGMb的相互作用。针对PD-L2/RGMb的抗体可以在生态失调的情况下恢复对PD-1阻断的反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
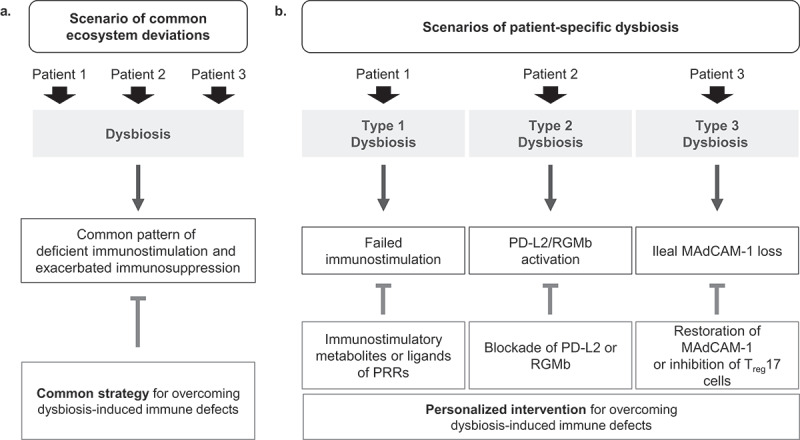
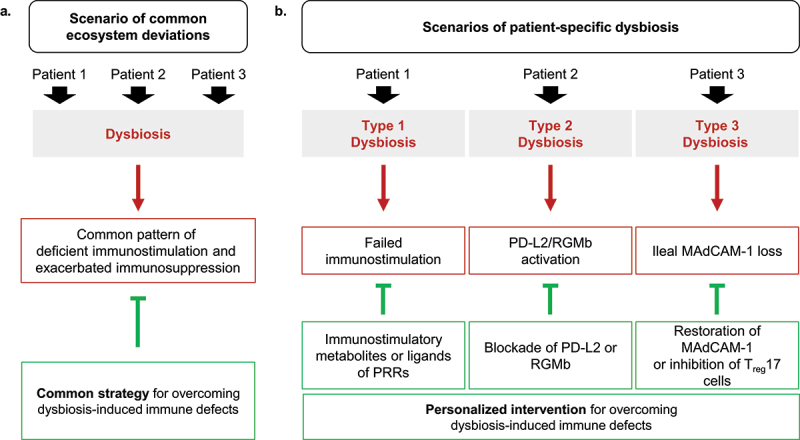
Microbiota-associated immunotherapy resistance caused by deficient PD-L2 - RGMb signaling.
In a recent paper in Nature, Park et al. propose a mechanism through which intestinal dysbiosis compromises the efficacy of immunotherapy targeting the PD-L1/PD-1 interaction. Dysbiosis may upregulate a pair of checkpoint molecules, i.e. PD-L2 interacting with RGMb. Antibodies targeting PD-L2/RGMb can restore responses to PD-1 blockade in the context of dysbiosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncoimmunology
ONCOLOGY-IMMUNOLOGY
CiteScore
12.80
自引率
2.80%
发文量
276
期刊介绍:
Tumor immunology explores the natural and therapy-induced recognition of cancers, along with the complex interplay between oncogenesis, inflammation, and immunosurveillance. In response to recent advancements, a new journal, OncoImmunology, is being launched to specifically address tumor immunology. The field has seen significant progress with the clinical demonstration and FDA approval of anticancer immunotherapies. There's also growing evidence suggesting that many current chemotherapeutic agents rely on immune effectors for their efficacy.
While oncologists have historically utilized chemotherapeutic and radiotherapeutic regimens successfully, they may have unwittingly leveraged the immune system's ability to recognize tumor-specific antigens and control cancer growth. Consequently, immunological biomarkers are increasingly crucial for cancer prognosis and predicting chemotherapy efficacy. There's strong support for combining conventional anticancer therapies with immunotherapies. OncoImmunology will welcome high-profile submissions spanning fundamental, translational, and clinical aspects of tumor immunology, including solid and hematological cancers, inflammation, and both innate and acquired immune responses.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


