新型 rAAV 向量介导的鞘内 HGF 给药对伴有 TDP-43 病理的 ALS 运动皮层的神经免疫调节有影响
IF 4.6
3区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
基于重组腺相关病毒(rAAV)的基因疗法为肌萎缩性脊髓侧索硬化症(ALS)等罕见疾病的治疗提供了巨大的机会。在这里,我们介绍了一种新型载体系统的产生、特性鉴定和利用,这种载体系统能在EF-1α启动子和牛生长激素(bGH)多聚(A)序列的作用下表达肝细胞生长因子(HGF)的活性形式,并且通过鞘内注射有效。HGF在促进运动神经元存活方面的作用已有大量报道。因此,我们研究了鞘内注射 HGF 是否会对 ALS 最常见的病理之一:TDP-43 病理产生影响。星形胶质细胞增多、微胶质细胞增生和进行性上运动神经元缺失是 ALS 在具有 TDP-43 病理学的运动皮质中造成的重要后果。我们发现可通过鞘内注射调节大脑皮层,表达 HGF 可减少运动皮层的星形胶质细胞和小胶质细胞增多,并有助于恢复正在发生的 UMN 退化。我们的研究结果不仅引入了一种治疗渐冻人症的新型病毒载体,还证明了通过鞘内注射病毒可调节运动皮质,而且 HGF 治疗可有效减少伴有 TDP-43 病理的渐冻人症运动皮质中的星形胶质细胞和小胶质细胞。本文章由计算机程序翻译,如有差异,请以英文原文为准。
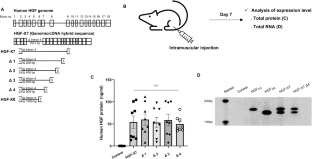
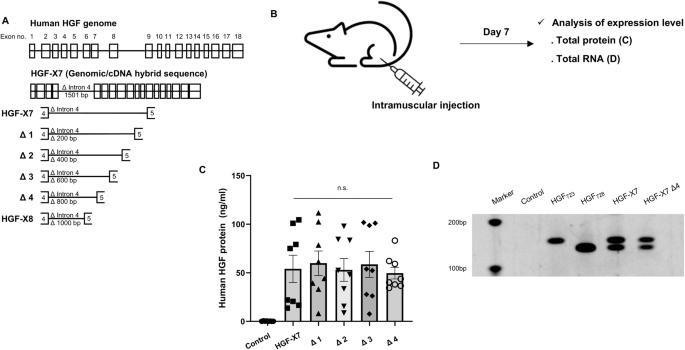
Novel rAAV vector mediated intrathecal HGF delivery has an impact on neuroimmune modulation in the ALS motor cortex with TDP-43 pathology
Recombinant adeno-associated virus (rAAV)-based gene therapies offer an immense opportunity for rare diseases, such as amyotrophic lateral sclerosis (ALS), which is defined by the loss of the upper and the lower motor neurons. Here, we describe generation, characterization, and utilization of a novel vector system, which enables expression of the active form of hepatocyte growth factor (HGF) under EF-1α promoter with bovine growth hormone (bGH) poly(A) sequence and is effective with intrathecal injections. HGF’s role in promoting motor neuron survival had been vastly reported. Therefore, we investigated whether intrathecal delivery of HGF would have an impact on one of the most common pathologies of ALS: the TDP-43 pathology. Increased astrogliosis, microgliosis and progressive upper motor neuron loss are important consequences of ALS in the motor cortex with TDP-43 pathology. We find that cortex can be modulated via intrathecal injection, and that expression of HGF reduces astrogliosis, microgliosis in the motor cortex, and help restore ongoing UMN degeneration. Our findings not only introduce a novel viral vector for the treatment of ALS, but also demonstrate modulation of motor cortex by intrathecal viral delivery, and that HGF treatment is effective in reducing astrogliosis and microgliosis in the motor cortex of ALS with TDP-43 pathology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Gene Therapy
医学-生化与分子生物学
CiteScore
9.70
自引率
2.00%
发文量
67
审稿时长
4-8 weeks
期刊介绍:
Gene Therapy covers both the research and clinical applications of novel therapeutic techniques based on a genetic component. Over the last few decades, significant advances in technologies ranging from identifying novel genetic targets that cause disease through to clinical studies, which show therapeutic benefit, have elevated this multidisciplinary field to the forefront of modern medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


