XC001(encoberminogene rezmadenovec)基因疗法在大鼠体内的安全性和生物分布:心血管疾病的一种潜在疗法。
IF 4.6
3区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
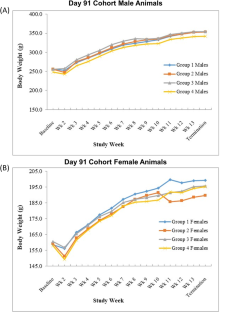
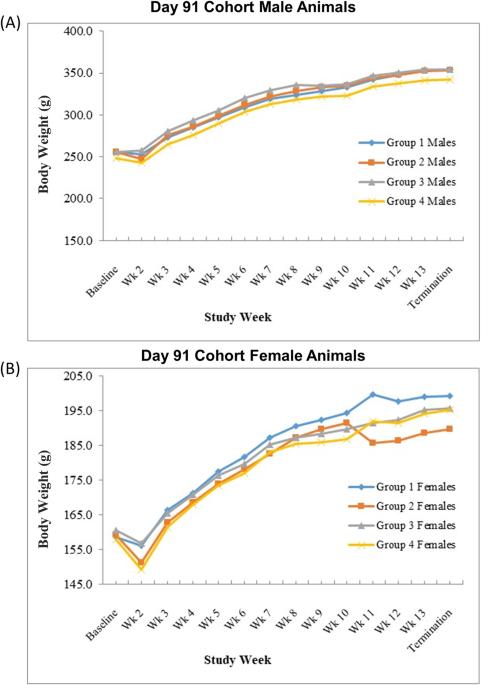
腺病毒介导的基因疗法有望治疗难治性心绞痛等心血管疾病。然而,需要对免疫原性和载体从注射靶组织扩散的潜在问题进行评估。本研究旨在评估 XC001 的安全性和生物分布。XC001 是一种复制缺陷型腺病毒血清型 5 载体,表达多种异构体的人血管内皮生长因子(VEGF)。动物接受缓冲制剂或剂量不断增加的 XC001(1 × 107、2.5 × 108 或 2.5 × 109 病毒颗粒)。根据生前参数(一般健康状况、体重、临床病理学、血清心肌肌钙蛋白 I、血浆血管内皮生长因子和尸体解剖),没有发现任何临床问题。第8天,心肌内注射XC001与剂量相关,注射部位出现左心室心肌炎症,第30天炎症消退。任何时候都未在血液中检测到XC001 DNA,但在第8天时,注射部位周围出现了XC001 DNA,在脾脏、肝脏和肺部也出现了XC001 DNA,但程度较轻,在心脏和脾脏中以低水平持续存在,至少持续到第91天。这些研究结果表明,心肌内注射XC001可用于人体研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Safety and biodistribution of XC001 (encoberminogene rezmadenovec) gene therapy in rats: a potential therapy for cardiovascular diseases
Adenovirus-mediated gene therapy holds promise for the treatment of cardiovascular diseases such as refractory angina. However, potential concerns around immunogenicity and vector dissemination from the target injected tissue require evaluation. This study was undertaken to evaluate the safety and biodistribution of XC001, a replication-deficient adenovirus serotype 5 vector expressing multiple isoforms of human vascular endothelial growth factor (VEGF), following direct administration into normal rat myocardium. Animals received the buffer formulation or increasing doses of XC001 (1 × 107, 2.5 × 108 or 2.5 × 109 viral particles). Based on in-life parameters (general health, body weights, clinical pathology, serum cardiac troponin I, plasma VEGF, and gross necropsy), there were no findings of clinical concern. On Day 8, intramyocardial administration of XC001 was associated with dose-related, left ventricular myocardial inflammation at injection sites, resolving by Day 30. XC001 DNA was not detected in blood at any time but was present at Day 8 around the site of injection and to a much lesser extent in the spleen, liver, and lungs, persisting at low levels in the heart and spleen until at least Day 91. These findings demonstrate that intramyocardial injection of XC001 is supported for use in human studies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Gene Therapy
医学-生化与分子生物学
CiteScore
9.70
自引率
2.00%
发文量
67
审稿时长
4-8 weeks
期刊介绍:
Gene Therapy covers both the research and clinical applications of novel therapeutic techniques based on a genetic component. Over the last few decades, significant advances in technologies ranging from identifying novel genetic targets that cause disease through to clinical studies, which show therapeutic benefit, have elevated this multidisciplinary field to the forefront of modern medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


