Genetic variation at innate and adaptive immune genes – contrasting patterns of differentiation and local adaptation in a wild gull
IF 3.1
2区 生物学
Q2 ECOLOGY
引用次数: 0
Abstract
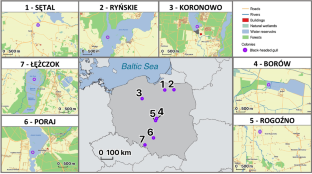
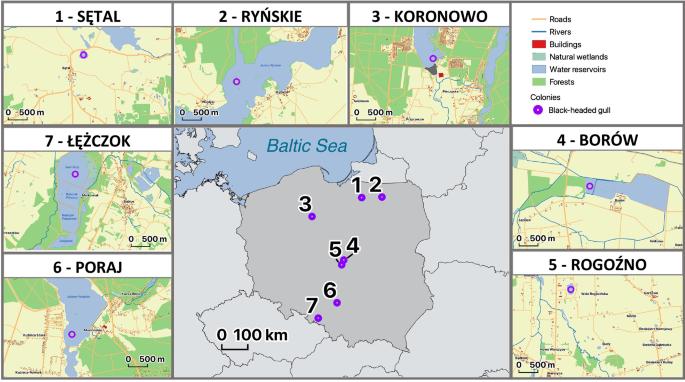
Immunogenetic variation in natural vertebrate populations is expected to respond to spatial and temporal fluctuations in pathogen assemblages. While spatial heterogeneity in pathogen-driven selection enhances local immunogenetic adaptations and population divergence, different immune genes may yield contrasting responses to the environment. Here, we investigated population differentiation at the key pathogen recognition genes of the innate and adaptive immune system in a colonial bird species, the black-headed gull Chroicocephalus ridibundus. We assessed genetic variation at three toll-like receptor (TLR) genes (innate immunity) and the major histocompatibility complex (MHC) class I and II genes (adaptive immunity) in gulls from seven colonies scattered across Poland. As expected, we found much greater polymorphism at the MHC than TLRs. Population differentiation at the MHC class II, but not MHC-I, was significantly stronger than at neutral microsatellite loci, suggesting local adaptation. This could reflect spatial variation in the composition of extracellular parasite communities (e.g., helminths), possibly driven by sharp differences in habitat structure between colonies. Despite contrasting patterns of population differentiation, both MHC classes showed similar regimes of diversifying selection. Some significant population differentiation was also observed at TLRs, suggesting that innate immune receptors may respond to fine-scale spatial variation in pathogen pressure, although this pattern could have been enhanced by drift. Our results suggested that local adaptation at the pathogen recognition immune genes can be maintained at relatively small or moderate spatial scales in species with high dispersal potential and they highlighted the complexity of immunogenetic responses of animals to heterogeneous environments.
先天免疫基因和适应性免疫基因的遗传变异——野生海鸥分化和局部适应的对比模式。
自然脊椎动物种群的免疫遗传变异预计会对病原体组合的空间和时间波动做出反应。虽然病原体驱动的选择的空间异质性增强了局部免疫遗传适应和群体差异,但不同的免疫基因可能对环境产生不同的反应。在这里,我们研究了一种群体性鸟类——黑头鸥——先天和适应性免疫系统的关键病原体识别基因的群体分化。我们评估了来自波兰七个群体的海鸥的三个toll样受体(TLR)基因(先天免疫)和主要组织相容性复合体(MHC)I类和II类基因(适应性免疫)的遗传变异。正如预期的那样,我们发现MHC的多态性比TLR大得多。MHC II类(而非MHC-I)的群体分化明显强于中性微卫星位点,表明局部适应。这可能反映了细胞外寄生虫群落(如蠕虫)组成的空间变化,可能是由群落之间栖息地结构的巨大差异驱动的。尽管种群分化模式不同,但两个MHC类别都表现出相似的多样化选择机制。在TLRs中也观察到一些显著的群体分化,这表明先天免疫受体可能对病原体压力的精细空间变化做出反应,尽管这种模式可能因漂移而增强。我们的研究结果表明,在具有高传播潜力的物种中,病原体识别免疫基因的局部适应可以保持在相对较小或中等的空间尺度上,并突出了动物对异质环境的免疫遗传反应的复杂性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Heredity
生物-进化生物学
CiteScore
7.50
自引率
2.60%
发文量
84
审稿时长
4-8 weeks
期刊介绍:
Heredity is the official journal of the Genetics Society. It covers a broad range of topics within the field of genetics and therefore papers must address conceptual or applied issues of interest to the journal''s wide readership
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


