Signal Detection in Pharmacovigilance: A Review of Informatics-driven Approaches for the Discovery of Drug-Drug Interaction Signals in Different Data Sources
Abstract
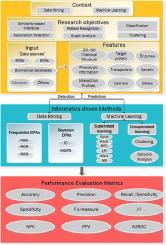
The objective of this article is to review the application of informatics-driven approaches in the pharmacovigilance field with focus on drug-drug interaction (DDI) safety signal discovery using various data sources. Signal can be a new safety information or new aspect to already known adverse drug reaction which is possibly causally related to a medication/medications that warrants further investigation to accept or refute. Signals can be detected from different data sources such as spontaneous reporting system, scientific literature, biomedical databases and electronic health records. This review is substantiated based on the fact that DDIs are contributing to a public health problem represented in 6-30% adverse drug event occurrences. In this article, we review informatics-driven approaches applied by authors focusing on DDI signal detection using different data sources. The aim of this article is not to laboriously survey all PV literature. As an alternative, we discussed informatics-driven methods used to discover DDI signals and various data sources reinforced with instances of studies from PV literature. The adoption of informatics-driven approaches can complement and optimize the practice of safety signal detection. However, further researches should be carried out to evaluate the efficiency of those approaches and to address the limitations of external validation, implementation and adoption in real clinical environments and by the regulatory bodies.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


