Knockdown of Yap attenuates TAA-induced hepatic fibrosis by interaction with hedgehog signals
Abstract
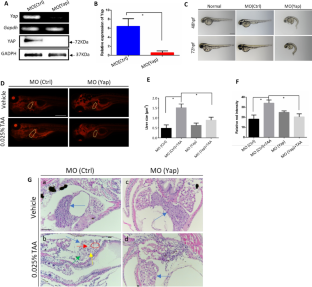
Liver fibrosis is an aberrant wound healing response to tissue injury characterized by excessive extracellular matrix deposition and loss of normal liver architecture. Hepatic stellate cells (HSCs) activation is regards to be the major process in liver fibrogenesis which is dynamic and reversible. Both Hippo signaling core factor Yap and Hedgehog (Hh) signaling promote HSCs transdifferentiation thereby regulating the repair process of liver injury. However, the molecular function of YAP and the regulation between Yap and Hh during fibrogenesis remain uncertain. In this study, the essential roles of Yap in liver fibrosis were investigated. Yap was detected to be increased in liver fibrotic tissue by the thioacetamide (TAA)-induced zebrafish embryonic and adult models. Inhibition of Yap by both embryonic morpholino interference and adult's inhibitor treatment was proved to alleviate TAA-induced liver lesions by and histology and gene expression examination. Transcriptomic analysis and gene expression detection showed that Yap and Hh signaling pathway have a cross talking upon TAA-induced liver fibrosis. In addition, TAA induction promoted the nuclear colocalization of YAP and Hh signaling factor GLI2α. This study demonstrates that Yap and Hh play synergistic protective roles in liver fibrotic response and provides new theoretical insight concerning the mechanisms of fibrosis progression.


 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


