Transcriptional changes associated with apoptosis and type I IFN underlie the early interaction between Besnoitia besnoiti tachyzoites and monocyte-derived macrophages
Abstract
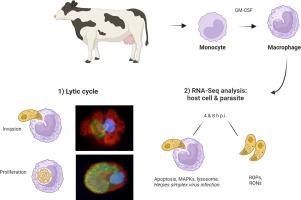
Besnoitia besnoiti-infected bulls may develop severe systemic clinical signs and orchitis that may ultimately cause sterility during the acute infection. Macrophages might play a relevant role in pathogenesis of the disease and the immune response raised against B. besnoiti infection. This study aimed to dissect the early interaction between B. besnoiti tachyzoites and primary bovine monocyte-derived macrophages in vitro. First, the B. besnoiti tachyzoite lytic cycle was characterized. Next, dual transcriptomic profiling of B. besnoiti tachyzoites and macrophages was conducted at early infection (4 and 8 h p.i.) by high-throughput RNA sequencing. Macrophages inoculated with heat-killed tachyzoites (MO-hkBb) and non-infected macrophages (MO) were used as controls. Besnoitia besnoiti was able to invade and proliferate in macrophages. Upon infection, macrophage activation was demonstrated by morphological and transcriptomic changes. Infected macrophages were smaller, round and lacked filopodial structures, which might be associated with a migratory phenotype demonstrated in other apicomplexan parasites. The number of differentially expressed genes (DEGs) increased substantially during infection. In B. besnoiti-infected macrophages (MO-Bb), apoptosis and mitogen-activated protein kinase (MAPK) pathways were regulated at 4 h p.i., and apoptosis was confirmed by TUNEL assay. The Herpes simplex virus 1 infection pathway was the only significantly enriched pathway in MO-Bb at 8 h p.i. Relevant DEGs of the Herpes simplex virus 1 infection (IFNα) and the apoptosis pathways (CHOP-2) were also significantly regulated in the testicular parenchyma of naturally infected bulls. Furthermore, the parasite transcriptomic analysis revealed DEGs mainly related to host cell invasion and metabolism. These results provide a deep overview of the earliest macrophage modulation by B. besnoiti that may favour parasite survival and proliferation in a specialized phagocytic immune cell. Putative parasite effectors were also identified.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


