Assessment of Medication Errors Among Anesthesia Clinicians in Saudi Arabia: A Cross-Sectional Survey Study.
引用次数: 2
Abstract
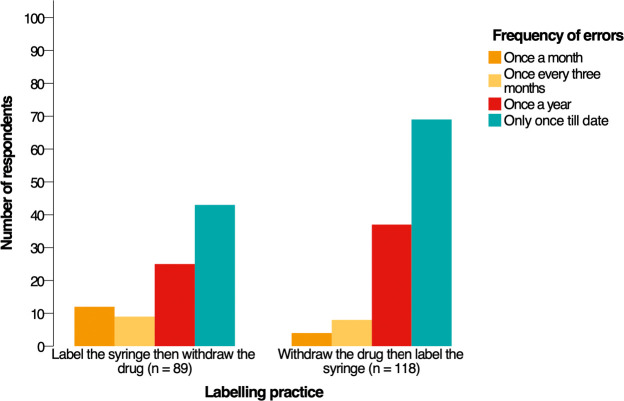
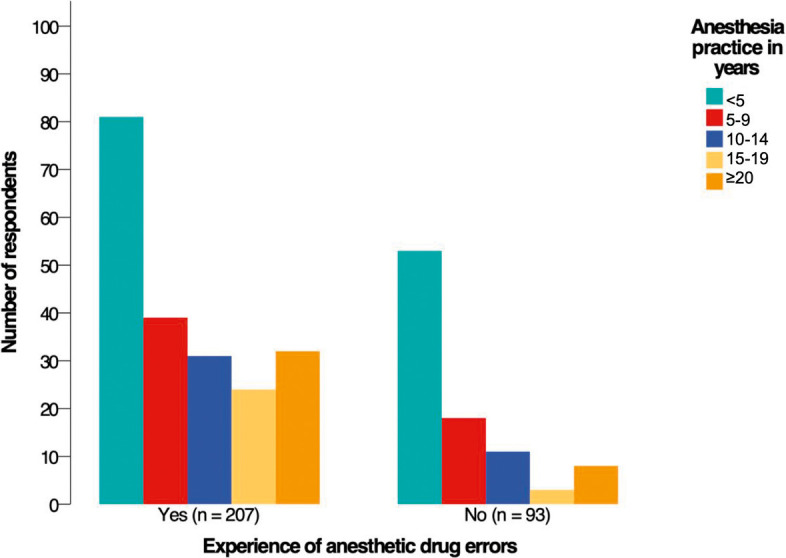
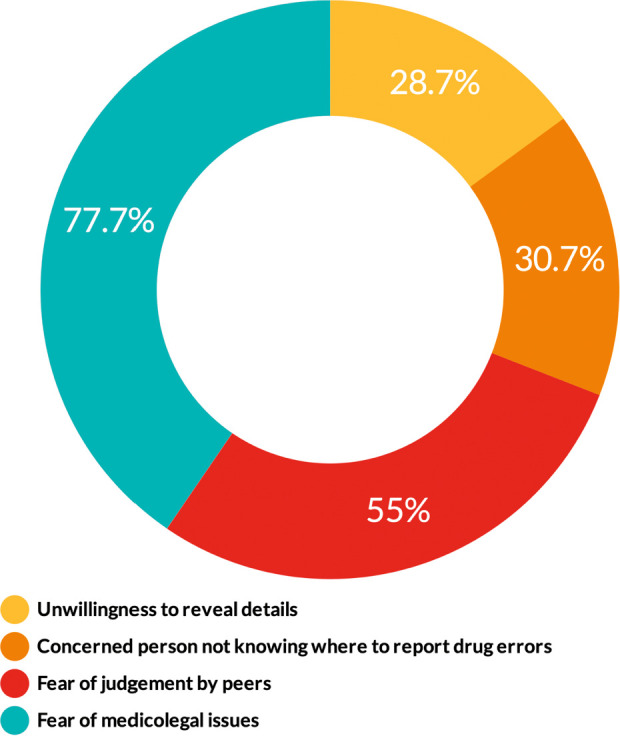
Introduction Anesthetic drugs are prepared and administrated without referral to the pharmacy or other medical departments. We aimed to assess the occurrence of anesthetic drug errors in Saudi Arabia. We also determined the contributing factors, reporting strategies, and clinicians' opinions of the preventive measures. Methods We conducted a cross-sectional web-based survey study using a validated tool. A total of 300 anesthesia clinicians completed the survey (146 anesthesiologists and 154 anesthesia technology specialists). We measured descriptive statistics to describe the demographic characteristics and performed inferential statistics to examine associations and differences. Results Sixty-nine percent of respondents had experienced an anesthetic drug error at least once in their career. The two primary factors that caused drug errors were haste (60.3%) and heavy workload (60.3%). On syringe labeling, 56.3% withdrew the drug then labeled the syringe, and 43.7% labeled the syringe then withdrew the drug. The chi-square test revealed that clinicians who labeled the syringe first then withdrew the drug made errors more frequently (p = 0.036). The test also showed that clinicians with less experience had committed more errors (p = 0.015). On reporting drug errors, 77.7% of respondents identified the fear of medicolegal issues as the most common barrier to reporting errors. Respondents believed that double-checking the medication and color-coded syringe labels were the most effective strategies to reduce errors (82% and 64%, respectively). The Mann-Whiney U test revealed significant differences between the two specialties about their opinions of the preventive measures. Conclusions There was a high occurrence rate of anesthetic drug errors in Saudi Arabia. Policymakers need to unify the syringe-labeling practice, and future research needs to focus on what makes a nonpunitive culture to encourage reporting errors.
沙特阿拉伯麻醉临床医生用药错误的评估:一项横断面调查研究。
简介:麻醉药物的制备和使用无需转介到药房或其他医疗部门。我们的目的是评估沙特阿拉伯麻醉药物错误的发生率。我们还确定了影响因素、报告策略和临床医生对预防措施的意见。方法:我们使用经过验证的工具进行了一项基于网络的横断面调查研究。共有300名麻醉临床医生完成了调查(146名麻醉医师和154名麻醉技术专家)。我们测量了描述性统计来描述人口统计学特征,并进行了推论统计来检查关联和差异。结果:69%的受访者在其职业生涯中至少经历过一次麻醉药物错误。导致用药差错的两个主要因素是匆忙(60.3%)和工作繁重(60.3%)。在注射器标签方面,56.3%的人先退药再给注射器贴标签,43.7%的人先给注射器贴标签再退药。卡方检验显示,先给注射器贴上标签后再取药的临床医生更容易出错(p = 0.036)。该测试还显示,经验较少的临床医生犯了更多的错误(p = 0.015)。在报告药物错误方面,77.7%的受访者认为对医学法律问题的恐惧是报告错误最常见的障碍。受访者认为,反复检查药物和颜色编码的注射器标签是减少错误的最有效策略(分别为82%和64%)。Mann-Whiney U测试揭示了两个专业对预防措施的看法存在显著差异。结论:沙特阿拉伯麻醉药品差错发生率较高。政策制定者需要统一注射器标签的做法,未来的研究需要关注是什么使非惩罚性文化鼓励报告错误。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


