Effect of tubulin self-association on GTP hydrolysis and nucleotide exchange reactions
Abstract
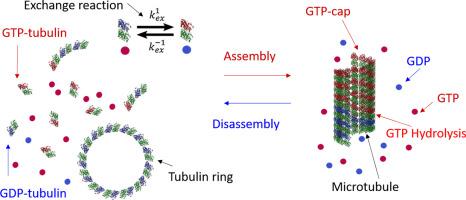
We investigated how the self-association of isolated tubulin dimers affects the rate of GTP hydrolysis and the equilibrium of nucleotide exchange. Both reactions are relevant for microtubule (MT) dynamics. We used HPLC to determine the concentrations of GDP and GTP and thereby the GTPase activity of SEC-eluted tubulin dimers in assembly buffer solution, free of glycerol and tubulin aggregates. When GTP hydrolysis was negligible, the nucleotide exchange mechanism was studied by determining the concentrations of tubulin-free and tubulin-bound GTP and GDP. We observed no GTP hydrolysis below the critical conditions for MT assembly (either below the critical tubulin concentration and/or at low temperature), despite the assembly of tubulin 1D curved oligomers and single-rings, showing that their assembly did not involve GTP hydrolysis. Under conditions enabling spontaneous slow MT assembly, a slow pseudo-first-order GTP hydrolysis kinetics was detected, limited by the rate of MT assembly. Cryo-TEM images showed that GTP-tubulin 1D oligomers were curved also at 36 °C. Nucleotide exchange depended on the total tubulin concentration and the molar ratio between tubulin-free GDP and GTP. We used a thermodynamic model of isodesmic tubulin self-association, terminated by the formation of tubulin single-rings to determine the molar fractions of dimers with exposed and buried nucleotide exchangeable sites (E-sites). Our analysis shows that the GDP to GTP exchange reaction equilibrium constant was an order-of-magnitude larger for tubulin dimers with exposed E-sites than for assembled dimers with buried E-sites. This conclusion may have implications on the dynamics at the tip of the MT plus end.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


