Second-Generation Atroposelective Synthesis of KRAS G12C Covalent Inhibitor GDC-6036
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 6
Abstract
A chromatography-free asymmetric synthesis of GDC-6036 (1) was achieved via a highly atroposelective Negishi coupling of aminopyridine 5 and quinazoline 6b catalyzed by 0.5 mol % [Pd(cin)Cl]2 and 1 mol % (R,R)-Chiraphite to afford the key intermediate (Ra)-3. An alkoxylation of (Ra)-3 with (S)-N-methylprolinol (4) and a global deprotection generates the penultimate heterobiaryl intermediate 2. A controlled acrylamide installation by stepwise acylation/sulfone elimination and final adipate salt formation and crystallization delivered high-purity GDC-6036 (1).
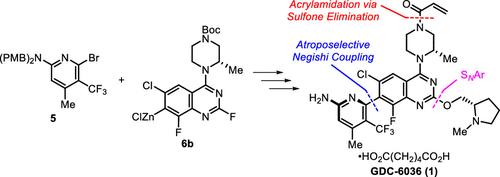
第二代KRAS G12C共价抑制剂GDC-6036的水选择性合成
通过氨基吡啶5和喹唑啉6b的根岸偶联,以0.5 mol % [Pd(cin)Cl]2和1 mol % (R,R)-Chiraphite催化得到关键中间体(Ra)-3,实现了GDC-6036(1)的无色谱不对称合成。(Ra)-3与(S)- n -甲基脯氨酸醇(4)的烷氧基化和全局去保护产生了倒数第二的杂环芳烃中间体2。通过逐步酰化/砜消除和最终己二酸盐形成和结晶控制丙烯酰胺的安装,可获得高纯度的GDC-6036(1)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


