Lemniscular carbon nanohoops with contiguous conjugation from planar chiral [2.2]paracyclophane: influence of the regioselective synthesis on topological chirality†
IF 7.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 2
Abstract
We report herein the regioselective synthesis of all-carbon lemniscular nanohoops bis-po-CC and bis-pm-TC by the rational control of ring closures at the different positions of planar chiral tetrasubstituted [2.2]paracyclophane. Topological analyses reveal that bis-pm-TC is topologically chiral while bis-po-CC is topologically achiral. X-ray crystal analysis demonstrates that bis-pm-TC adopts a lemniscular conformation with a contiguous conjugation. CD and CPL measurements further reveal that the chiroptical properties of bis-pm-TC are obviously different from those of bis-po-CC due to their different topological chiralities.
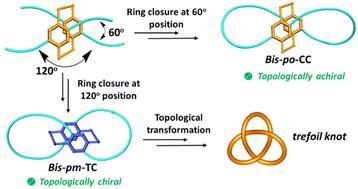
平面手性[2.2]副环烷连续共轭的环状碳纳米环:区域选择性合成对拓扑手性的影响
本文报道了通过合理控制平面手性四取代[2.2]副环环烷不同位置的闭环,区域选择性合成了全碳环状纳米环bis-po-CC和bis-pm-TC。拓扑分析表明,双聚丙烯腈具有拓扑手性,而双聚丙烯腈具有拓扑非手性。x -射线晶体分析表明,铋-pm- tc具有连续共轭的lemniscle构象。CD和CPL测量进一步表明,由于其不同的拓扑手性,双-pm- tc与双-po- cc的手性性质明显不同。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Science
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
14.40
自引率
4.80%
发文量
1352
审稿时长
2.1 months
期刊介绍:
Chemical Science is a journal that encompasses various disciplines within the chemical sciences. Its scope includes publishing ground-breaking research with significant implications for its respective field, as well as appealing to a wider audience in related areas. To be considered for publication, articles must showcase innovative and original advances in their field of study and be presented in a manner that is understandable to scientists from diverse backgrounds. However, the journal generally does not publish highly specialized research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


