In Situ Allene Formation via Alkyne Tautomerization to Promote [4 + 2]-Cycloadditions with a Pendant Alkyne or Nitrile
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 1
Abstract
We have explored the net-[4 + 2]-cycloadditions of a variety of aryl (or alkenyl) alkynes. Tautomerization via base-catalyzed alkyne-to-allene isomerization produces a transient allene, which undergoes stepwise cyclization with not only a pendant alkyne but also a nitrile. The operative mechanisms for these reactions were studied by density functional theory and compared with the slower thermal cyclization of the precursor alkyne.
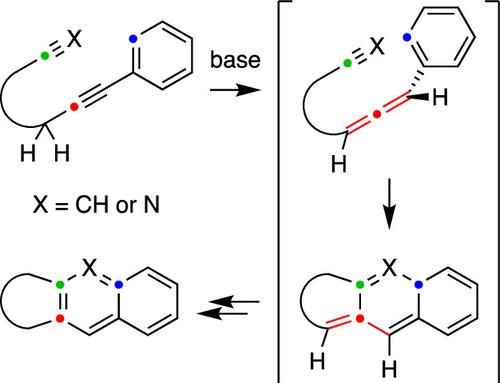
通过炔互变异构原位生成烯促进[4 + 2]-环与垂链炔或腈的加成
我们探索了各种芳基(或烯基)炔的净[4 + 2]环加成。通过碱催化的炔-烯异构化产生瞬时烯,它不仅与一个垂链炔而且与一个腈进行逐步环化。用密度泛函理论研究了这些反应的作用机理,并与前体炔的慢热环化反应进行了比较。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


