The Direct Conversion of Esters to Ketones Enabled by a Traceless Activating Group
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 1
Abstract
We report here the design and development of a method for the single-step conversion of esters to ketones with simple reagents. The selective transformation of esters to ketones, rather than tertiary alcohols, is made possible by the use of a transient sulfinate group on the nucleophile that activates the adjacent carbon toward deprotonation to form a carbanion that adds to the ester, followed by a second deprotonation to prevent further addition. The resulting dianion undergoes spontaneous fragmentation of the SO2 group upon quenching with water to reveal the ketone product.
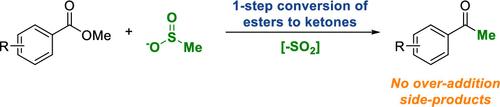
无迹激活基团使酯类直接转化为酮类
我们在这里报告了一种方法的设计和发展,以简单的试剂一步转化为酮酯。酯选择性转化为酮,而不是叔醇,是通过在亲核试剂上使用一个瞬时亚磺酸基来激活相邻的碳去质子化,形成一个碳离子,加到酯上,然后是第二次去质子化,以防止进一步的加成。所得的离子在水淬后,SO2基团发生自发碎裂,露出酮产物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


