Ni/Photoredox-Catalyzed C(sp2)–C(sp3) Cross-Coupling of Alkyl Pinacolboronates and (Hetero)Aryl Bromides
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 3
Abstract
Utilizing quinoline as a mild, catalytic additive, broadly applicable conditions for the Ni/photoredox-catalyzed C(sp2)–C(sp3) cross-coupling of (hetero)aryl bromides and alkyl pinacolboronate esters were developed, which can be applied to both batch and flow reactions. In addition to primary benzylic nucleophiles, both stabilized and nonstabilized secondary alkyl boronic esters are effective coupling partners. Density functional theory calculations suggest that alkyl radical generation occurs from an alkyl-B(pin)-quinoline complex, which may proceed via an energy transfer process.
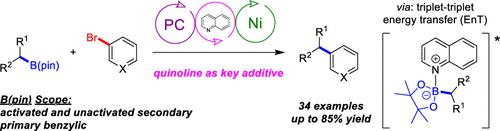
Ni/光氧化还原催化的C(sp2) -C (sp3)交偶联烷基松酰硼酸酯和(杂)芳基溴
利用喹啉作为温和的催化添加剂,开发了镍/光氧化还原催化(杂)芳基溴与烷基松酸酯的C(sp2) -C (sp3)交联反应的广泛适用条件,该条件既可用于间歇反应,也可用于流动反应。除了伯苯基亲核试剂外,稳定的和非稳定的仲烷基硼酯都是有效的偶联伙伴。密度泛函理论计算表明,烷基自由基的生成是由烷基- b(针)-喹啉配合物产生的,这可能是通过能量转移过程进行的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


