ROS induced the Rab26 promoter hypermethylation to promote cigarette smoking-induced airway epithelial inflammation of COPD through activation of MAPK signaling
Abstract
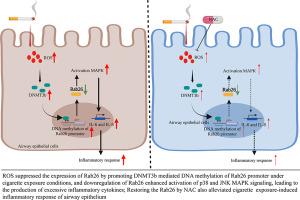
Cigarette smoking (CS) exposure-induced airway inflammatory responses drive the occurrence and development of emphysema and chronic obstructive pulmonary disease (COPD). However, its precise mechanisms have not been fully elucidated. In this study, we explore the role of Rab26 in CS exposure modulating the inflammatory response of airway epithelium and the novel mechanism of CS exposure regulation Rab26. These data showed that CS exposure and H2O2 (a type of ROS) suppressed the expression of Rab26 and increased the expression of DNMT3b in vivo and in vitro. GEO data analysis found the level of Rab26 was decreased in the lung tissue of COPD patients. CSE-induced ROS promoted DNA methylation of the Rab26 promoter and inhibited its promoter activity by elevating the DNMT3b level. Antioxidants N-Acetyl-l-cysteine (NAC), 5-Aza-2′-deoxycytidine (5-AZA) (DNA methylation inhibitor) and DNMT3B siRNA alleviated CSE's inhibitory effect on Rab26 expression in vitro. Importantly, NAC alleviated the improved expression of Rab26 and reduced DNMT3B expression, in the airway of smoking exposure as well as attenuated the inflammatory response in vivo. Overexpression of Rab26 attenuated CSE-induced production of inflammatory mediators through part inactivation of p38 and JNK MAPK. On the contrary, silencing Rab26 enhanced p38 and JNK activation and aggravated inflammatory response. These findings suggest that ROS-mediated Rab26 promoter hypermethylation is a critical step in cigarette smoking-induced airway epithelial inflammatory response. Restoring Rab26 in the airway epithelium might be a potential strategy for treating airway inflammation and COPD.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


