CP-25 enhances OAT1-mediated absorption of methotrexate in synoviocytes of collagen-induced arthritis rats
IF 6.9
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Organic anion transporter 1 (OAT1) plays a major role in mediating the absorption, distribution and excretion of drugs and other xenobiotics in the human body. In this study we explored the OAT1 status in rheumatoid arthritis (RA) patients and arthritic animals and its role in regulating the anti-arthritic activity of methotrexate (MTX). We showed that OAT1 expression was significantly downregulated in synovial tissues from RA patients compared with that in the control patients. In collagen-induced arthritis (CIA) rats, synovial OAT1 expression was significantly decreased compared with the control rats. In synoviocytes isolated from CIA rats, PGE2 (0.003–1.75 μM) dose-dependently downregulated OAT1 expression, resulting in decreased absorption of MTX. Silencing OAT1 in synoviocytes caused a 43.7% reduction in the uptake of MTX. Furthermore, knockdown of OAT1 impaired MTX-induced inhibitory effects on the viability and migration of synoviocytes isolated from CIA rats. Moreover, injection of OAT1-shRNA into articular cavity of CIA rats significantly decreased synovial OAT1 expression and impaired the anti-arthritic action of MTX, while injection of lentivirus containing OAT1 sequences led to the opposite results. Interestingly, we found that paeoniflorin-6’-O-benzene sulfonate (CP-25) upregulated OAT1 expression both in vitro and in vivo and promoted MTX uptake by synoviocytes via regulating OAT1 expression and function. Taken together, OAT1 plays a major role in regulating MTX uptake by synoviocytes and the anti-arthritic activity of MTX. OAT1 is downregulated in RA and CIA rats, which can be improved by CP-25.
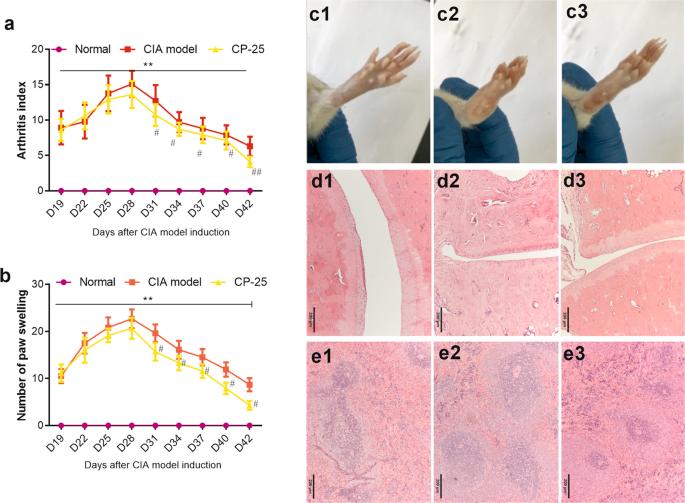
CP-25 可增强胶原诱导的关节炎大鼠滑膜细胞对 OAT1 介导的甲氨蝶呤的吸收
有机阴离子转运体 1(OAT1)在介导药物和其他异种生物在人体内的吸收、分布和排泄方面发挥着重要作用。在这项研究中,我们探讨了类风湿性关节炎(RA)患者和关节炎动物的 OAT1 状态及其在调节甲氨蝶呤(MTX)抗关节炎活性中的作用。我们发现,与对照组患者相比,OAT1 在 RA 患者滑膜组织中的表达明显下调。与对照组大鼠相比,胶原诱导的关节炎(CIA)大鼠滑膜 OAT1 的表达明显下降。在分离自 CIA 大鼠的滑膜细胞中,PGE2(0.003-1.75 μM)剂量依赖性地下调 OAT1 的表达,导致 MTX 的吸收减少。沉默滑膜细胞中的 OAT1 可使 MTX 的吸收减少 43.7%。此外,敲除 OAT1 会削弱 MTX 诱导的对 CIA 大鼠滑膜细胞活力和迁移的抑制作用。此外,将 OAT1-shRNA 注入 CIA 大鼠的关节腔可显著降低滑膜 OAT1 的表达,并削弱 MTX 的抗关节炎作用,而注入含有 OAT1 序列的慢病毒则会导致相反的结果。有趣的是,我们发现芍药苷-6'-O-苯磺酸盐(CP-25)能在体外和体内上调 OAT1 的表达,并通过调节 OAT1 的表达和功能促进滑膜细胞对 MTX 的吸收。综上所述,OAT1 在调节滑膜细胞对 MTX 的吸收和 MTX 的抗关节炎活性方面发挥着重要作用。OAT1在RA和CIA大鼠中表达下调,CP-25可改善这种情况。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Pharmacologica Sinica
医学-化学综合
CiteScore
15.10
自引率
2.40%
发文量
4365
审稿时长
2 months
期刊介绍:
APS (Acta Pharmacologica Sinica) welcomes submissions from diverse areas of pharmacology and the life sciences. While we encourage contributions across a broad spectrum, topics of particular interest include, but are not limited to: anticancer pharmacology, cardiovascular and pulmonary pharmacology, clinical pharmacology, drug discovery, gastrointestinal and hepatic pharmacology, genitourinary, renal, and endocrine pharmacology, immunopharmacology and inflammation, molecular and cellular pharmacology, neuropharmacology, pharmaceutics, and pharmacokinetics. Join us in sharing your research and insights in pharmacology and the life sciences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


