Analisi di Budget Impact della formulazione depot di buprenorfina a rilascio prolungato per la gestione di pazienti affetti da disturbo da uso di oppiacei.
IF 0.4
Q4 HEALTH CARE SCIENCES & SERVICES
引用次数: 1
Abstract
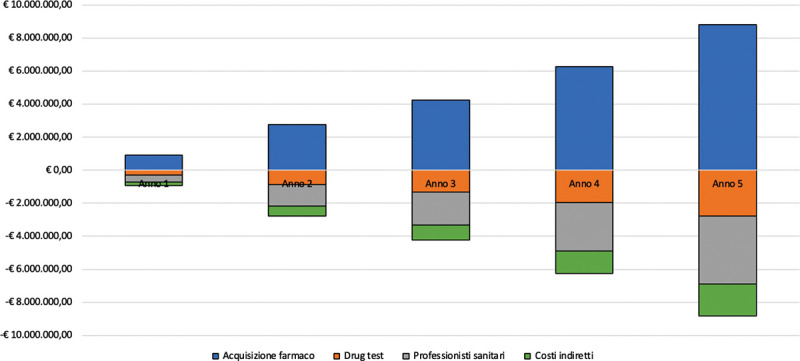
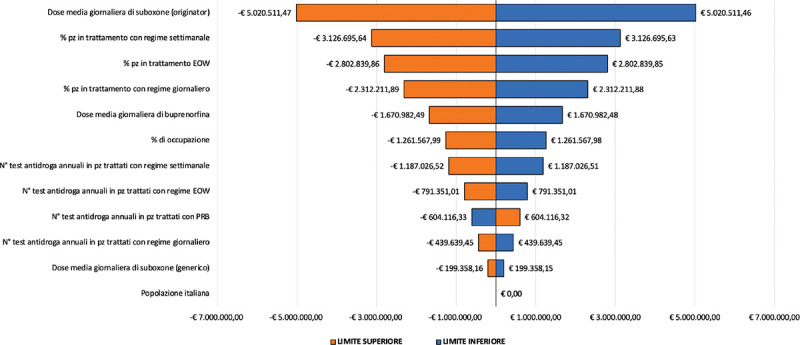
Budget Impact Analysis of prolonged-release buprenorphine depot-formulation for the management of patients affected by opioid use disorder Background: Opioid use disorder (OUD) is a disorder associated with significant rate of morbidity and mortality. Frequent clinic attendance for supervised consumption of sublingual buprenorphine is common. Prolonged-release buprenorphine (PRB) allows a management based on weekly or monthly subcutaneous injections, thus limiting the burdens of clinic attendance and the risks associated with sublingual formulations. Objective: To determine the price level of PRB that allows to obtain a neutral impact from the point of view of the economic resources absorbed, in comparison with the alternatives currently available in the Italian context for the management of patients suffering from OUD. Methods: The analysis assumes a daily PRB cost of € 8.526 (neutral cost). The analysis aims to determine the economic impact associated with the introduction of PRB in the Italian context for the management of OUD patients. Results are expressed in terms of differential resources absorbed in the alternative scenarios. A one-way sensitivity analysis was also carried out to test the robustness of the results. Results: The introduction of PRB implies an increase in the drug acquisition costs over the 5-year time horizon of € 23,016,194.61: such costs are fully compensated by the other cost driver considered in the analysis (drug tests provided, health professionals’ time destined to the provision of the treatment, indirect costs, for savings equal to € 7,255,602.59, € 10,714,320.08 and € 5,046,271.94 respectively) demonstrating its effectiveness in particular by an organizational point of view. Lower price levels for PRB would imply significant savings for the SSN. Conclusions: PRB resulted to be associated to a lower level of resources’ absorption in the Italian sector as compared with the available alternatives thus allowing to re-allocate health founds to other fields of the care sector ensuring greater safety for patients and a decreased misuse and diversion rate.
长期释放丁丙诺啡配方的预算影响分析
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Global & Regional Health Technology Assessment
HEALTH CARE SCIENCES & SERVICES-
CiteScore
0.80
自引率
20.00%
发文量
27
审稿时长
8 weeks
期刊介绍:
Global & Regional Health Technology Assessment (GRHTA) is a peer-reviewed, open access journal which aims to promote health technology assessment and economic evaluation, enabling choices among alternative therapeutical paths or procedures with different clinical and economic outcomes. GRHTA is a unique journal having three different editorial boards who focus on their respective geographical expertise.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


