Myosin V facilitates polarised E‐cadherin secretion
IF 2.5
3区 生物学
Q3 CELL BIOLOGY
引用次数: 5
Abstract
E‐cadherin has a fundamental role in epithelial tissues by providing cell–cell adhesion. Polarised E‐cadherin exocytosis to the lateral plasma membrane is central for cell polarity and epithelial homeostasis. Loss of E‐cadherin secretion compromises tissue integrity and is a prerequisite for metastasis. Despite this pivotal role of E‐cadherin secretion, the transport mechanism is still unknown. Here we identify Myosin V as the motor for E‐cadherin secretion. Our data reveal that Myosin V and F‐actin are required for the formation of a continuous apicolateral E‐cadherin belt, the zonula adherens. We show by live imaging how Myosin V transports E‐cadherin vesicles to the plasma membrane, and distinguish two distinct transport tracks: an apical actin network leading to the zonula adherens and parallel actin bundles leading to the basal‐most region of the lateral membrane. E‐cadherin secretion starts in endosomes, where Rab11 and Sec15 recruit Myosin V for transport to the zonula adherens. We also shed light on the endosomal sorting of E‐cadherin by showing how Rab7 and Snx16 cooperate in moving E‐cadherin into the Rab11 compartment. Thus, our data help to understand how polarised E‐cadherin secretion maintains epithelial architecture and prevents metastasis.
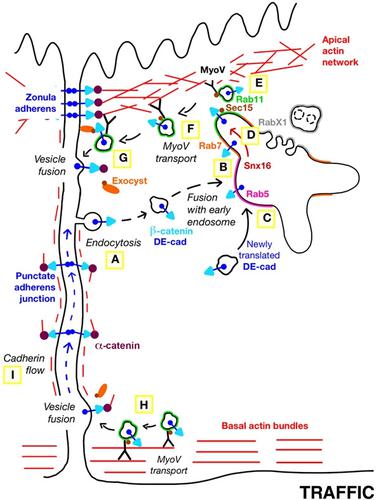
肌凝蛋白V促进E -钙粘蛋白的极化分泌
E‐钙粘蛋白通过提供细胞-细胞粘附在上皮组织中起着重要作用。极化的E -钙粘蛋白胞外分泌到侧质膜对细胞极性和上皮稳态至关重要。E -钙粘蛋白分泌的缺失损害了组织的完整性,是转移的先决条件。尽管E‐钙粘蛋白分泌具有关键作用,但其转运机制仍不清楚。在这里,我们确定肌凝蛋白V是E‐钙粘蛋白分泌的马达。我们的数据显示,肌凝蛋白V和F - actin是形成连续的顶端外侧E -钙粘蛋白带所必需的,小带粘附。我们通过实时成像展示了肌凝蛋白V如何将E -钙粘蛋白囊泡运输到质膜,并区分了两种不同的运输路径:顶端肌动蛋白网络通向小带粘附体,平行肌动蛋白束通向外侧膜的最基底区域。E‐钙粘蛋白分泌始于核内体,Rab11和Sec15招募肌凝蛋白V运输到小带粘附体。我们还通过展示Rab7和Snx16如何合作将E - cadherin移动到Rab11室来阐明E - cadherin的内体分选。因此,我们的数据有助于理解极化E -钙粘蛋白分泌如何维持上皮结构并防止转移。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Traffic
生物-细胞生物学
CiteScore
8.10
自引率
2.20%
发文量
50
审稿时长
2 months
期刊介绍:
Traffic encourages and facilitates the publication of papers in any field relating to intracellular transport in health and disease. Traffic papers span disciplines such as developmental biology, neuroscience, innate and adaptive immunity, epithelial cell biology, intracellular pathogens and host-pathogen interactions, among others using any eukaryotic model system. Areas of particular interest include protein, nucleic acid and lipid traffic, molecular motors, intracellular pathogens, intracellular proteolysis, nuclear import and export, cytokinesis and the cell cycle, the interface between signaling and trafficking or localization, protein translocation, the cell biology of adaptive an innate immunity, organelle biogenesis, metabolism, cell polarity and organization, and organelle movement.
All aspects of the structural, molecular biology, biochemistry, genetics, morphology, intracellular signaling and relationship to hereditary or infectious diseases will be covered. Manuscripts must provide a clear conceptual or mechanistic advance. The editors will reject papers that require major changes, including addition of significant experimental data or other significant revision.
Traffic will consider manuscripts of any length, but encourages authors to limit their papers to 16 typeset pages or less.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


