Highly enantioselective organocatalysis of the Michael addition of benzyloxyacetaldehyde to nitroolefins
Q2 Chemistry
引用次数: 1
Abstract
A novel category of di(N,N-dimethylbenzylamine)prolinol silyl ether catalyst, which when used in conjunction with an acidic co-catalyst, generates an ammonium salt supported organocatalyst. This catalytic system is shown to be very effective for the Michael reaction of benzyloxyacetaldehyde and various nitroolefins in isopropanol. Excellent enantioselectivities (up to 99%) and diastereoselectivities (syn/anti of 75:25) and short reaction times were obtained. The presence of the bulky OTMS group combined with the presence of two large N,N-dimethylbenzyl ammonium ion groups accounts for the effectiveness of this catalytic system.
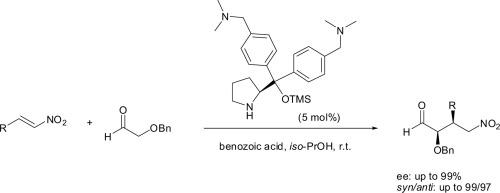
苯氧乙醛对硝基烯烃加成反应的高对映选择性有机催化
一类新型二(N,N-二甲基苄胺)脯氨醇硅基醚催化剂,当与酸性助催化剂结合使用时,生成铵盐负载型有机催化剂。该催化体系对异丙醇中苯氧乙醛和各种硝基烯烃的Michael反应非常有效。具有优异的对映选择性(高达99%)和非对映选择性(正/反为75:25),反应时间短。庞大的OTMS基团的存在加上两个大的N,N-二甲基苄基铵离子基团的存在说明了该催化体系的有效性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron, asymmetry
化学-无机化学与核化学
CiteScore
4.70
自引率
0.00%
发文量
0
审稿时长
1 months
期刊介绍:
Cessation. Tetrahedron: Asymmetry presents experimental or theoretical research results of outstanding significance and timeliness on asymmetry in organic, inorganic, organometallic and physical chemistry, as well as its application to related disciplines, especially bio-organic chemistry.
The journal publishes critical reviews, original research articles and preliminary communications dealing with all aspects of the chemical, physical and theoretical properties of non-racemic organic and inorganic materials and processes. Topics relevant to the journal include: the physico-chemical and biological properties of enantiomers; strategies and methodologies of asymmetric synthesis; resolution; chirality recognition and enhancement; analytical techniques for assessing enantiomeric purity and the unambiguous determination of absolute configuration; and molecular graphics and modelling methods for interpreting and predicting asymmetric phenomena. Papers describing the synthesis or properties of non-racemic molecules will be required to include a separate statement in the form of a Stereochemistry Abstract, for publication in the same issue, of the criteria used for the assignment of configuration and enantiomeric purity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


