Enantioselective Synthesis of Medium-Sized-Ring Lactones via Iridium-Catalyzed Z-Retentive Asymmetric Allylic Substitution Reaction
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 13
Abstract
Medium-sized rings are important structural units, but their synthesis, especially in a highly enantioselective manner, has been a great challenge. Herein we report an enantioselective synthesis of medium-sized-ring lactones by an iridium-catalyzed Z-retentive asymmetric allylic substitution reaction. The reaction features mild conditions and a broad substrate scope. Various eight- to 11-membered-ring lactones can be afforded in moderate to excellent yields (up to 88%) and excellent enantioselectivity (up to 99% ee). The utilization of both Z-allyl precursors and an Ir catalyst is critical for the medium-sized-ring formation.
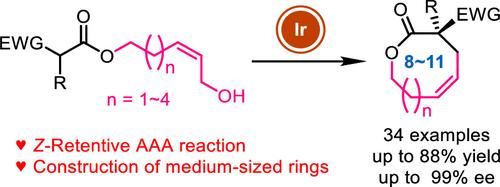
用铱催化的z -保留不对称烯丙基取代反应对映选择性合成中环内酯
中型环是重要的结构单元,但它们的合成,特别是以高度对映选择性的方式合成,一直是一个巨大的挑战。本文报道了一种铱催化的保持z的不对称烯丙基取代反应的中环内酯的对映选择性合成。该反应条件温和,底物范围广。各种8 - 11元环内酯可以提供中等至优异的产率(高达88%)和优异的对映体选择性(高达99% ee)。z -烯丙基前驱体和Ir催化剂的使用对中等大小环的形成至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


