Response to Crudele et al. Commentary on Gudin et al. "Comparing the Effect of Tampering on the Oral Pharmacokinetic Profiles of Two Extended-Release Oxycodone Formulations with Abuse-Deterrent Properties".
Pain Medicine: The Official Journal of the American Academy of Pain Medicine
Pub Date : 2017-05-01
DOI:10.1093/pm/pnw279
引用次数: 0
对Crudele等人的回应。对Gudin等人的评论。“篡改对两种具有防滥用特性的羟考酮缓释制剂口服药代动力学的影响比较”。
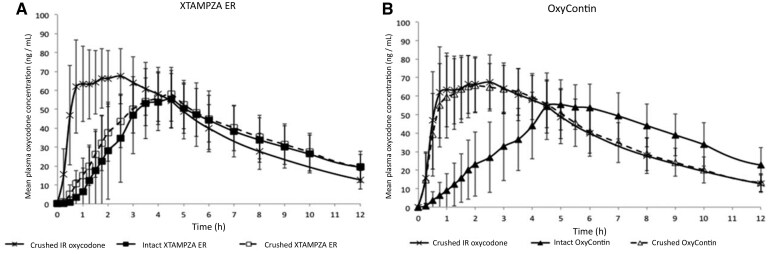
尊敬的编辑,我们感谢Crudele和同事花时间阅读我们的出版物“比较篡改对两种具有抗滥用特性的羟考酮缓释制剂的口服药代动力学特征的影响”[1],其中比较了操纵Xtampza缓释(ER)和操纵重新配制的奥施康定的药代动力学(PK)特征。我们非常感谢编辑们给我们一个回复他们评论的机会。Crudele指出,这篇论文“暗示这些PK结果得到了比较药效学(药物喜欢效应)或人类滥用潜力研究数据的支持,而事实并非如此。”参考文献中的研究(Gudin et al. 2015)[1]没有收集比较药效学数据,因为操纵处理组的PK结果差异非常明显,并且是独立的:粉碎的Xtampza ER(羟考酮)的PK谱与完整的Xtampza ER具有生物等效性(图1A)[1]。这与压碎后的奥施康定(盐酸羟考酮)的情况形成对比,后者与压碎后的奥施康定完全不同,与压碎后的羟考酮片具有生物等效性(图1B)[1]。参考研究中提供的数据最近在第二项研究中得到了重复[2],并且是…
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


