Asymmetric synthesis of tert-butyl ((1R,4aR,8R,8aR)-1-hydroxyoctahydro-1H-isochromen-8-yl)carbamate
Q2 Chemistry
引用次数: 2
Abstract
The asymmetric synthesis of methyl (E)-4-((1R,2S,3R)-3-amino-2-((E)-2-methoxycarbonyl-eten-1-yl)cyclohexyl)but-2-enoate 14 has been achieved from dimethyl (2E,7E)-nona-2,7-dienedioate 2. A key step is the asymmetric synthesis of 1-hydroxyoctahydro-1H-isochromene derivative 5 whose X-ray analysis corroborated the stereochemistry of the new stereocenters. The asymmetric synthesis of the isochromenyl acetate derivative 11 shows the potential of this methodology for fused cyclohexanic system heterocyclic synthesis.
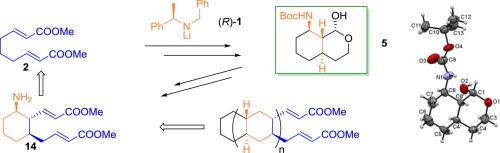
不对称合成叔丁基((1r,4a R, 8r,8a R)-1-羟基辛氢-1 H -异色胺-8-基)氨基甲酸酯
以二甲基(2E,7E)-壬基-2,7-二烯二酸酯2为原料,合成了甲基(E)-4-((1R,2S,3R)-3-氨基-2-((E)-2-甲氧基羰基-烯-1-基)环己基)-2-烯酸酯14。关键一步是不对称合成1-羟基八氢- 1h -等色胺衍生物5,其x射线分析证实了新立体中心的立体化学性质。不对称合成醋酸异铬戊酯衍生物11显示了该方法用于融合环己烷体系杂环合成的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron, asymmetry
化学-无机化学与核化学
CiteScore
4.70
自引率
0.00%
发文量
0
审稿时长
1 months
期刊介绍:
Cessation. Tetrahedron: Asymmetry presents experimental or theoretical research results of outstanding significance and timeliness on asymmetry in organic, inorganic, organometallic and physical chemistry, as well as its application to related disciplines, especially bio-organic chemistry.
The journal publishes critical reviews, original research articles and preliminary communications dealing with all aspects of the chemical, physical and theoretical properties of non-racemic organic and inorganic materials and processes. Topics relevant to the journal include: the physico-chemical and biological properties of enantiomers; strategies and methodologies of asymmetric synthesis; resolution; chirality recognition and enhancement; analytical techniques for assessing enantiomeric purity and the unambiguous determination of absolute configuration; and molecular graphics and modelling methods for interpreting and predicting asymmetric phenomena. Papers describing the synthesis or properties of non-racemic molecules will be required to include a separate statement in the form of a Stereochemistry Abstract, for publication in the same issue, of the criteria used for the assignment of configuration and enantiomeric purity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


