Dual chiral silver catalyst in the synthetic approach to the core of hepatitis C virus inhibitor GSK 625433 using enantioselective 1,3-dipolar cycloaddition of azomethine ylides and electrophilic alkenes
Q2 Chemistry
引用次数: 5
Abstract
The asymmetric 1,3-dipolar cycloaddition of an imino ester 5 with tert-butyl acrylate is catalyzed by a dual chiral silver(I) complex formed from a chiral phosphoramidite 14 and the chiral silver(I) binolphosphate (R)-17. This reaction is selected to achieve the synthesis of enantiomerically enriched key structures to access the third generation of GSK HCV inhibitors. The scope of this dual chiral catalytic system is analyzed by employing different imino esters and dipolarophiles, and also compared with the same cycloaddition reactions performed with the chiral phosphoramidite 14·AgClO4 complex.
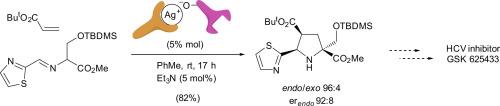
双手性银催化剂在丙型肝炎病毒抑制剂GSK 625433的核心合成方法中采用对映选择性1,3-偶极环加成法将偶氮亚胺酰基与亲电烯烃结合
由手性磷酰胺14和手性二磷酸银(R)-17形成的双手性银(I)配合物催化了亚胺酯5与丙烯酸叔丁酯的不对称1,3-偶极环加成反应。选择该反应是为了合成对映体富集的关键结构,从而获得第三代GSK HCV抑制剂。通过使用不同的亚胺酯和亲偶极试剂分析了该双手性催化体系的作用范围,并与手性磷酰胺14·AgClO4配合物进行了相同的环加成反应进行了比较。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron, asymmetry
化学-无机化学与核化学
CiteScore
4.70
自引率
0.00%
发文量
0
审稿时长
1 months
期刊介绍:
Cessation. Tetrahedron: Asymmetry presents experimental or theoretical research results of outstanding significance and timeliness on asymmetry in organic, inorganic, organometallic and physical chemistry, as well as its application to related disciplines, especially bio-organic chemistry.
The journal publishes critical reviews, original research articles and preliminary communications dealing with all aspects of the chemical, physical and theoretical properties of non-racemic organic and inorganic materials and processes. Topics relevant to the journal include: the physico-chemical and biological properties of enantiomers; strategies and methodologies of asymmetric synthesis; resolution; chirality recognition and enhancement; analytical techniques for assessing enantiomeric purity and the unambiguous determination of absolute configuration; and molecular graphics and modelling methods for interpreting and predicting asymmetric phenomena. Papers describing the synthesis or properties of non-racemic molecules will be required to include a separate statement in the form of a Stereochemistry Abstract, for publication in the same issue, of the criteria used for the assignment of configuration and enantiomeric purity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


