Extracellular 20S proteasome secreted via microvesicles can degrade poorly folded proteins and inhibit Galectin‐3 agglutination activity
IF 2.5
3区 生物学
Q3 CELL BIOLOGY
引用次数: 0
Abstract
Proteasomes are major non‐lysosomal proteolytic complexes localized in the cytoplasm and in the nucleus of eukaryotic cells. Strikingly, high levels of extracellular proteasome have also been evidenced in the plasma (p‐proteasome) of patients with specific diseases. Here, we examined the process by which proteasomes are secreted, as well as their structural and functional features once in the extracellular space. We demonstrate that assembled 20S core particles are secreted by cells within microvesicles budding from the plasma membrane. Part of the extracellular proteasome pool is also free of membranes in the supernatant of cultured cells, and likely originates from microvesicles leakage. We further demonstrate that this free proteasome released by cells (cc‐proteasome for cell culture proteasome) possesses latent proteolytic activity and can degrade various extracellular proteins. Both standard (no immune‐subunits) and intermediate (containing some immune‐subunits) forms of 20S are observed. Moreover, we show that galectin‐3, which displays a highly disordered N‐terminal region, is efficiently cleaved by purified cc‐proteasome, without SDS activation, likely after its binding to PSMA3 (α7) subunit through its intrinsically disordered region. As a consequence, galectin‐3 is unable to induce red blood cells agglutination when preincubated with cc‐proteasome. These results highlight potential novel physio‐ and pathologic functions for the extracellular proteasome.
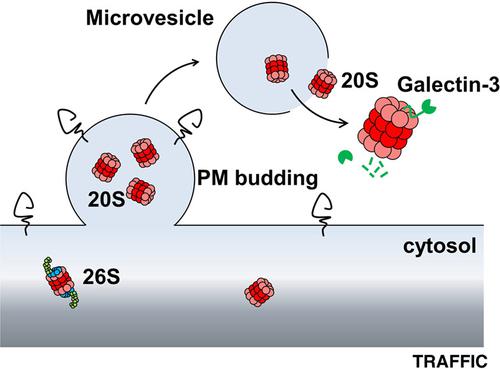
通过微泡分泌的胞外20S蛋白酶体可以降解折叠不良的蛋白质并抑制凝集素- 3的凝集活性
蛋白酶体是一种主要的非溶酶体蛋白水解复合物,存在于真核细胞的细胞质和细胞核中。引人注目的是,高水平的细胞外蛋白酶体也被证实存在于特定疾病患者的血浆(p -蛋白酶体)中。在这里,我们研究了蛋白酶体分泌的过程,以及它们在细胞外空间的结构和功能特征。我们证明组装的20S核心颗粒是由细胞质膜出芽的微泡内的细胞分泌的。部分细胞外蛋白酶体池在培养细胞的上清液中也无膜,可能来源于微泡渗漏。我们进一步证明,这种由细胞释放的游离蛋白酶体(cc -蛋白酶体为细胞培养蛋白酶体)具有潜在的蛋白水解活性,可以降解各种细胞外蛋白。标准型(无免疫亚基)和中间型(含一些免疫亚基)的20S均被观察到。此外,我们发现具有高度无序N端区域的凝集素- 3可以被纯化的cc -蛋白酶体有效地切割,而不需要SDS激活,这可能是在它通过其内在无序区域与PSMA3 (α7)亚基结合之后。因此,当与cc -蛋白酶体预孵育时,凝集素- 3不能诱导红细胞凝集。这些结果强调了细胞外蛋白酶体潜在的新的生理和病理功能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Traffic
生物-细胞生物学
CiteScore
8.10
自引率
2.20%
发文量
50
审稿时长
2 months
期刊介绍:
Traffic encourages and facilitates the publication of papers in any field relating to intracellular transport in health and disease. Traffic papers span disciplines such as developmental biology, neuroscience, innate and adaptive immunity, epithelial cell biology, intracellular pathogens and host-pathogen interactions, among others using any eukaryotic model system. Areas of particular interest include protein, nucleic acid and lipid traffic, molecular motors, intracellular pathogens, intracellular proteolysis, nuclear import and export, cytokinesis and the cell cycle, the interface between signaling and trafficking or localization, protein translocation, the cell biology of adaptive an innate immunity, organelle biogenesis, metabolism, cell polarity and organization, and organelle movement.
All aspects of the structural, molecular biology, biochemistry, genetics, morphology, intracellular signaling and relationship to hereditary or infectious diseases will be covered. Manuscripts must provide a clear conceptual or mechanistic advance. The editors will reject papers that require major changes, including addition of significant experimental data or other significant revision.
Traffic will consider manuscripts of any length, but encourages authors to limit their papers to 16 typeset pages or less.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


