Inhibition Effect of NaHCO3 on the Explosion of Mg–Al Alloy Powder
Abstract
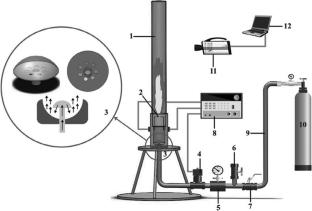
For explosion-proof and effective suppression measures in treating magnesium–aluminum alloy, it is of great importance to study the suppression of Mg–Al alloy dust explosions to prevent explosion disasters. The addition of inert solid substances to combustible dust is a measure aimed at preventing and reducing dust explosions. The explosion characteristics and flame propagation characteristics of Mg–Al alloy powder were studied using the Hartmann tube 20 L spherical explosion experimental system. The mechanism for removing sodium bicarbonate (NaHCO3) during the Mg–Al alloy powder explosion was further studied. The results show that the explosion pressure, the height of the deflagration flame, and the speed at which the flame propagates can be effectively reduced by increasing the percentage of NaHCO3. After the addition of 80% NaHCO3, the flame was suppressed, and the maximum explosion pressure decreased to less than 0.1 MPa, causing a 93% decrease in the maximum flame propagation speed. The process of suppressing NaHCO3 powder on magnesium aluminum alloy dust explosion is relatively complex, starting primarily with physical and chemical suppression. NaHCO3 realizes physical inhibition by reducing ambient temperature and oxygen concentration through the H2O and CO2 generated by decomposition. At the same time, through the cycle of NaO ↔ Na, the transformation from highly active oxygen to low active oxygen is realized. This reduces the activity of the explosive combustion response of Mg–Al alloy powder. At the same time, sodium ions can capture free radicals in explosive responses, reducing the number of free radicals in the reaction system and terminating the combustion reaction chain in advance. The research findings are of great importance for the safety of Mg–Al alloy production.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


