Targeting Aminoglycoside Acetyltransferase Activity of Mycobacterium tuberculosis (H37Rv) Derived Eis (Enhanced Intracellular Survival) Protein with Quercetin
Abstract
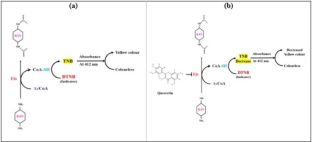
Eis (Enhanced intracellular survival) protein is an aminoglycoside acetyltransferase enzyme classified under the family – GNAT (GCN5-related family of N-acetyltransferases) secreted by Mycobacterium tuberculosis (Mtb). The enzymatic activity of Eis results in the acetylation of kanamycin, thereby impairing the drug’s action. In this study, we expressed and purified recombinant Eis (rEis) to determine the enzymatic activity of Eis and its potential inhibitor. Glide-enhanced precision docking was used to perform molecular docking with chosen ligands. Quercetin was found to interact Eis with a maximum binding affinity of -8.379 kcal/mol as compared to other ligands. Quercetin shows a specific interaction between the positively charged amino acid arginine in Eis and the aromatic ring of quercetin through π-cation interaction. Further, the effect of rEis was studied on the antibiotic activity of kanamycin A in the presence and absence of quercetin. It was observed that the activity of rEis aminoglycoside acetyltransferase decreased with increasing quercetin concentration. The results from the disk diffusion assay confirmed that increasing the concentration of quercetin inhibits the rEis protein activity. In conclusion, quercetin may act as a potential Eis inhibitor.


 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


