Nickel-Catalyzed Reductive Allylation of Aldehydes with Allylic Alcohols in the Presence of CO2
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
CO2-assisted and Ni-catalyzed direct reductive allylation of aldehydes utilizing allylic alcohols as allylic precursor has been reported. Various homoallyl alcohols could be synthesized in excellent yield with enhanced regioselectivity and stereoselectivity for alkyl- and aryl-substituted aldehydes under mild conditions. For different substrates, proper collocation of the catalytic precursor and ligand is crucial. Preliminary mechanistic studies supported the reaction pathway through a sequential allyl hydrocarbonate formation/allylnickelation/coordination insertion process by the Ni(I)/Ni(III) catalytic cycle, which has been proven by cyclic voltammetry analysis.
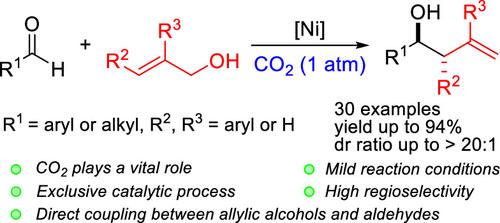
在CO2存在下,镍催化醛与烯丙醇的还原烯丙基化。
已经报道了利用烯丙醇作为烯丙基前体的CO2辅助和Ni催化的醛的直接还原烯丙基化。在温和的条件下,可以以优异的产率合成各种高烯丙醇,并提高了对烷基和芳基取代醛的区域选择性和立体选择性。对于不同的底物,催化前体和配体的适当配置至关重要。初步的机理研究支持了通过Ni(I)/Ni(III)催化循环依次形成烯丙基碳酸氢盐/烯丙基镍化/配位插入过程的反应途径,循环伏安法分析已经证明了这一点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


