Direct Asymmetric Reductive Amination
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 144
Abstract
Asymmetric reductive amination of β-keto amides catalyzed by the chiral catalyst Ru(OAc)2((R)-dm-segphos) produces unprotected β-amino amides with high yields and high enantioselectivities (94.7?99.5% ee). This “one-pot” methodology is general in substrate scope and has been successfully employed to produce sitagliptin with 99.5% ee and 91% assay yield. The excellent reaction efficiency is attributed to the remarkable tolerance to high concentrations of ammonium ion, the high chemoselectivity, and the high enantioselectivity (99.5% ee) of the Ru catalyst system.
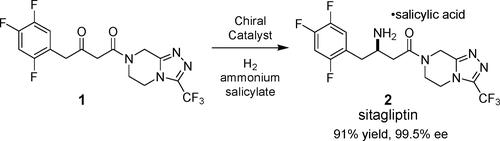
直接不对称还原胺化
手性催化剂Ru(OAc)2((R)-dm-segphos)催化β-酮酰胺的不对称还原胺化反应,得到了产率高、对映选择性高(94.7 ~ 99.5% ee)的无保护β-氨基酰胺。这种“一锅法”在底物范围内是通用的,并已成功地用于生产西格列汀,其纯度为99.5%,测定收率为91%。优异的反应效率归因于Ru催化剂体系对高浓度铵离子的耐受性、高化学选择性和高对映选择性(99.5% ee)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


