Safety, Tolerability, Pharmacokinetics and Quantitative Electroencephalography Assessment of ACD856, a Novel Positive Allosteric Modulator of Trk-Receptors Following Multiple Doses in Healthy Subjects
IF 7.8
3区 医学
Q1 CLINICAL NEUROLOGY
引用次数: 1
Abstract
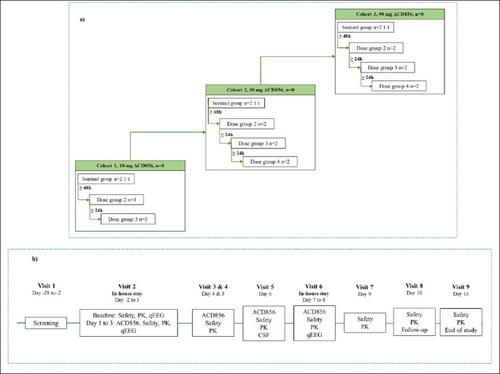
Background ACD856 is a positive allosteric modulator of tropomyosin receptor kinase (Trk) receptors which has shown to have pro-cognitive and anti-depressant-like effects in various animal models. It is currently in clinical development for the treatment of Alzheimer’s disease and other disorders where cognition is impaired and is also considered for indications such as depression or other neuropsychiatric diseases. ACD856 has a novel mechanism of action modulating the activity of the Trk-receptors, resulting in increased stimulation of the neurotrophin signaling pathways. Previous studies applying single intravenous and oral doses of ACD856 indicate that ACD856 is safe and well-tolerated by healthy volunteer subjects, and that it has suitable safety and pharmacokinetic properties for further clinical development. Objectives To investigate the safety and tolerability of 7 days of treatment with multiple ascending oral doses of ACD856 in healthy subjects, and to characterize its pharmacokinetic (PK) properties. In addition, pharmacodynamic effects of ACD856 using quantitative electroencephalography (qEEG) as an indicator for central target engagement were assessed. Design This was a prospective, phase I, double-blind, parallel-group, placebo-controlled, randomized study of the safety, tolerability, PK and pharmacodynamics of multiple ascending oral doses of ACD856 in healthy subjects. ACD856 or placebo were administered in 3 ascending dose cohorts of 8 subjects. Within each cohort, subjects were randomized to receive either ACD856 (n=6) or placebo (n=2). Setting The study was conducted at a First-in-Human unit in Sweden. Participants Twenty-four healthy male and female subjects. Intervention The study medication was administered as an oral solution, with ACD856 or the same contents without the active ingredient (placebo). The dose levels ranged from 10 mg to 90 mg. ACD856 was administered once daily for 7 days, targeting steady state. Measurements Safety and tolerability assessments included adverse events, laboratory, vital signs, 12-lead electrocardiogram (ECG), physical examination, assessment of stool frequency and questionnaires to assess symptoms of anxiety, depression, as well as suicidal ideation and behavior. In addition, cardiodynamic ECGs were extracted to evaluate cardiac safety. PK parameters were calculated based on measured concentrations of ACD856 in plasma, urine, and cerebrospinal fluid (CSF) samples. Metabolite profiling, characterization and analysis was performed based on and urine samples. qEEG was recorded for patients in the two highest dose cohorts (30 and 90 mg/day) as a pharmacodynamic assessment to explore central target engagement. Results Treatment with ACD856 was well tolerated with no serious adverse events. No treatment emergent or dose related trends were observed for any of the safety assessments. ACD856 was rapidly absorbed and reached maximum plasma exposure at 30 to 45 minutes after administration. Steady state was reached before Day 6, with an elimination half-life at steady state of approximately 20 hours. At steady state, ACD856 exhibited accumulation ratios for Cmax and AUC of approximately 1.6 and 1.9 respectively. The exposure, Cmax and AUC0-24, increased proportionally with the dose. There was no unchanged ACD856 detected in urine. The metabolic pattern in urine and plasma was similar, and in alignment with the metabolites observed in preclinical toxicology studies. The level of ACD856 measured in CSF at steady state increased with dose, indicating Central Nervous System (CNS) exposure at relevant levels for pharmacodynamic effects. ACD856 demonstrated significant dose-dependent treatment-associated changes on qEEG parameters. Specifically, increase of the relative theta power and decrease of the fast alpha and beta power was observed, leading to an acceleration of the delta+theta centroid and an increase in the theta/beta ratio. Conclusions ACD856 was well tolerated at the tested dose levels (10–90 mg/daily for 7 days) in healthy subjects. The compound has a robust pharmacokinetic profile, with rapid absorption and dose-dependent exposure. ACD856 was shown to pass the blood-brain-barrier, reach relevant exposure in the CNS and to induce dose-dependent treatment-related changes on qEEG parameters, indicating central target engagement.
新型trk受体正变构调节剂ACD856在健康受试者多剂量后的安全性、耐受性、药代动力学和定量脑电图评估
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Jpad-Journal of Prevention of Alzheimers Disease
CLINICAL NEUROLOGY-
自引率
7.80%
发文量
85
期刊介绍:
The JPAD « Journal of Prevention of Alzheimer’Disease » will publish reviews, original research articles and short reports to improve our knowledge in the field of Alzheimer prevention including : neurosciences, biomarkers, imaging, epidemiology, public health, physical cognitive exercise, nutrition, risk and protective factors, drug development, trials design, and heath economic outcomes.
JPAD will publish also the meeting abstracts from Clinical Trial on Alzheimer Disease (CTAD) and will be distributed both in paper and online version worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


