Everolimus and Malignancy after Solid Organ Transplantation: A Clinical Update.
IF 2.2
Q3 SURGERY
引用次数: 54
Abstract
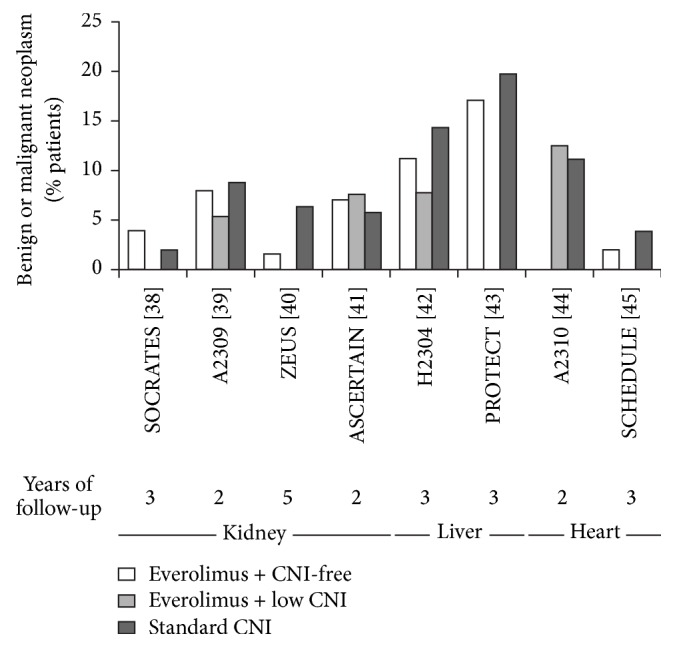
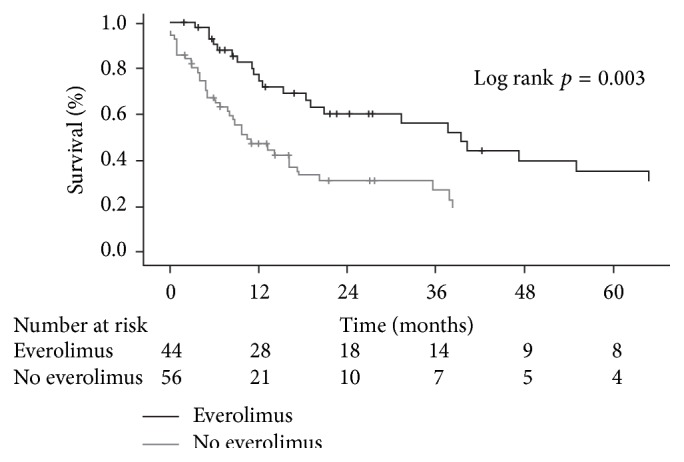
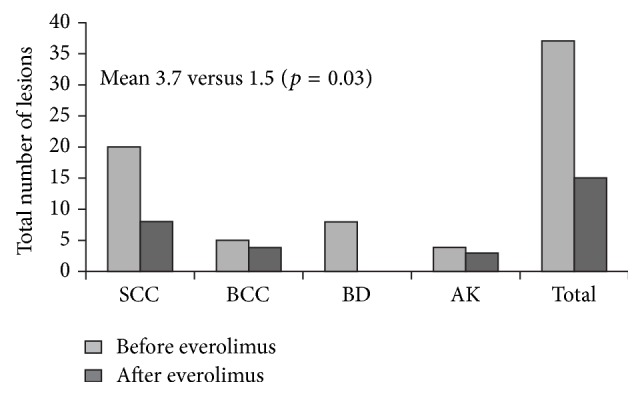
Malignancy after solid organ transplantation remains a major cause of posttransplant mortality. The mammalian target of rapamycin (mTOR) inhibitor class of immunosuppressants exerts various antioncogenic effects, and the mTOR inhibitor everolimus is licensed for the treatment of several solid cancers. In kidney transplantation, evidence from registry studies indicates a lower rate of de novo malignancy under mTOR inhibition, with some potentially supportive data from randomized trials of everolimus. Case reports and small single-center series have suggested that switch to everolimus may be beneficial following diagnosis of posttransplant malignancy, particularly for Kaposi's sarcoma and nonmelanoma skin cancer, but prospective studies are lacking. A systematic review has shown mTOR inhibition to be associated with a significantly lower rate of hepatocellular carcinoma (HCC) recurrence versus standard calcineurin inhibitor therapy. One meta-analysis has concluded that patients with nontransplant HCC experience a low but significant survival benefit under everolimus monotherapy, so far unconfirmed in a transplant population. Data are limited in heart transplantation, although observational data and case reports have indicated that introduction of everolimus is helpful in reducing the recurrence of skin cancers. Overall, it can be concluded that, in certain settings, everolimus appears a promising option to lessen the toll of posttransplant malignancy.
依维莫司与实体器官移植后的恶性肿瘤:临床进展。
实体器官移植后的恶性肿瘤仍然是移植后死亡的主要原因。哺乳动物靶向雷帕霉素(mTOR)抑制剂类免疫抑制剂具有多种抗原性作用,mTOR抑制剂依维莫司被许可用于治疗几种实体癌症。在肾移植中,来自注册研究的证据表明,mTOR抑制下的新发恶性肿瘤发生率较低,依维莫司随机试验的一些潜在支持数据。病例报告和小型单中心研究表明,移植后恶性肿瘤诊断后改用依维莫司可能是有益的,特别是卡波西肉瘤和非黑色素瘤皮肤癌,但缺乏前瞻性研究。一项系统综述显示,与标准钙调磷酸酶抑制剂治疗相比,mTOR抑制与肝细胞癌(HCC)复发率显著降低相关。一项荟萃分析得出结论,非移植性HCC患者在依维莫司单药治疗下生存率低但显著,迄今尚未在移植人群中得到证实。尽管观察数据和病例报告表明,依维莫司的引入有助于减少皮肤癌的复发,但在心脏移植方面的数据有限。总的来说,可以得出结论,在某些情况下,依维莫司似乎是一个有希望的选择,以减少移植后恶性肿瘤的死亡。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


