Martha E. Hay, Si Hui Wong, Sanjoy Mukherjee, Bryan W. Boudouris
下载PDF
{"title":"Controlling open-shell loading in norbornene-based radical polymers modulates the solid-state charge transport exponentially","authors":"Martha E. Hay, Si Hui Wong, Sanjoy Mukherjee, Bryan W. Boudouris","doi":"10.1002/polb.24406","DOIUrl":null,"url":null,"abstract":"<div>\n \n <p>Radical polymers are an emerging class of electronically active macromolecules; however, the fundamental mechanism by which charge is transferred in these polymers has yet to be established in full. To address this issue, well-defined norbornene-based nitroxide radical polymers were synthesized using the controlled ring-opening metathesis polymerization technique. These polymers were blended in solution with a quenched, electrically insulating hydroxylamine derivative to dilute the radical content of the system. Electron paramagnetic resonance spectroscopy data were used to characterize the radical content as well as to reveal that hydrogen atom transfer occurred between the open-shell and closed-shell polynorbornene derivatives when they were blended in solution. Using these platform macromolecules, we demonstrate that the systematic manipulation of the radical content in open-shell macromolecules leads to exponential changes in the macroscopic electrical conductivity. When coupled with the fact that these materials show a clear temperature-independent charge transport behavior, a picture emerges that charge transfer in radical polymers is dictated by a tunneling mechanism between localized donor and acceptor sites within the redox-active thin films. These results constitute the first experimental insight into the mechanism of solid-state electrical conduction in radical polymers, and this provides a design paradigm for open-shell macromolecular charge transport. © 2017 Wiley Periodicals, Inc. J. Polym. Sci., Part B: Polym. Phys. <b>2017</b>, <i>55</i>, 1516–1525</p>\n </div>","PeriodicalId":199,"journal":{"name":"Journal of Polymer Science Part A: Polymer Chemistry","volume":"55 20","pages":"1516-1525"},"PeriodicalIF":2.7020,"publicationDate":"2017-08-01","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://sci-hub-pdf.com/10.1002/polb.24406","citationCount":"22","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Journal of Polymer Science Part A: Polymer Chemistry","FirstCategoryId":"1","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/polb.24406","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"Materials Science","Score":null,"Total":0}
引用次数: 22
引用
批量引用
Abstract
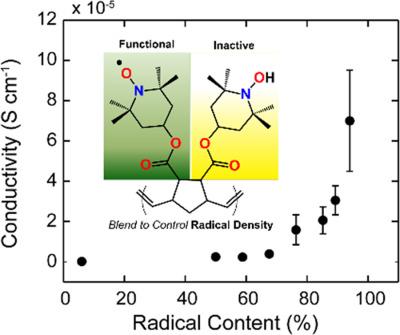
Radical polymers are an emerging class of electronically active macromolecules; however, the fundamental mechanism by which charge is transferred in these polymers has yet to be established in full. To address this issue, well-defined norbornene-based nitroxide radical polymers were synthesized using the controlled ring-opening metathesis polymerization technique. These polymers were blended in solution with a quenched, electrically insulating hydroxylamine derivative to dilute the radical content of the system. Electron paramagnetic resonance spectroscopy data were used to characterize the radical content as well as to reveal that hydrogen atom transfer occurred between the open-shell and closed-shell polynorbornene derivatives when they were blended in solution. Using these platform macromolecules, we demonstrate that the systematic manipulation of the radical content in open-shell macromolecules leads to exponential changes in the macroscopic electrical conductivity. When coupled with the fact that these materials show a clear temperature-independent charge transport behavior, a picture emerges that charge transfer in radical polymers is dictated by a tunneling mechanism between localized donor and acceptor sites within the redox-active thin films. These results constitute the first experimental insight into the mechanism of solid-state electrical conduction in radical polymers, and this provides a design paradigm for open-shell macromolecular charge transport. © 2017 Wiley Periodicals, Inc. J. Polym. Sci., Part B: Polym. Phys. 2017 , 55 , 1516–1525
控制降冰片烯基自由基聚合物的开壳负载可以以指数方式调节固态电荷输运
自由基聚合物是一类新兴的电活性高分子;然而,电荷在这些聚合物中转移的基本机制尚未完全确定。为了解决这一问题,采用可控开环复分解聚合技术合成了性能良好的降冰片烯基氮氧化物自由基聚合物。将这些聚合物与一种淬灭的电绝缘羟胺衍生物混合在溶液中,以稀释体系中的自由基含量。利用电子顺磁共振波谱数据表征了自由基的含量,揭示了开壳和闭壳聚降冰片烯衍生物在溶液中混合时发生了氢原子转移。利用这些平台大分子,我们证明了系统地操纵开壳大分子中的自由基含量会导致宏观电导率的指数变化。结合这些材料显示出与温度无关的电荷传输行为这一事实,我们可以看到自由基聚合物中的电荷转移是由氧化还原活性薄膜中局部供体和受体位点之间的隧道机制决定的。这些结果构成了对自由基聚合物中固态导电机制的第一个实验见解,并为开壳大分子电荷传输提供了一个设计范例。©2017 Wiley期刊公司j .变异较大。科学。B部分:Polym。物理学报,2017,55 (5):1516-1525
本文章由计算机程序翻译,如有差异,请以英文原文为准。

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


