Qin Yang, Qing Miao, Hui Chen, Duo Li, Yongfeng Luo, Joanne Chiu, Hong-Jun Wang, Michael Chuvanjyan, Michael S Parmacek, Wei Shi
下载PDF
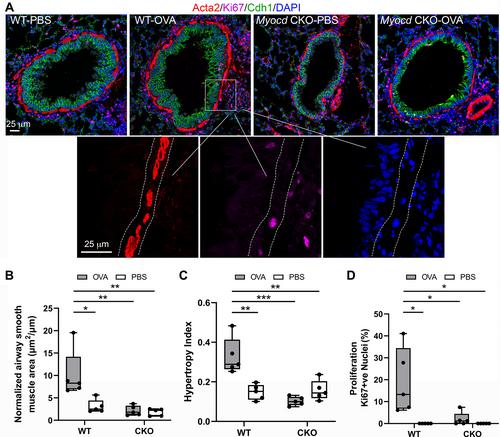
{"title":"Myocd regulates airway smooth muscle cell remodeling in response to chronic asthmatic injury","authors":"Qin Yang, Qing Miao, Hui Chen, Duo Li, Yongfeng Luo, Joanne Chiu, Hong-Jun Wang, Michael Chuvanjyan, Michael S Parmacek, Wei Shi","doi":"10.1002/path.6044","DOIUrl":null,"url":null,"abstract":"<p>Abnormal growth of airway smooth muscle cells is one of the key features in asthmatic airway remodeling, which is associated with asthma severity. The mechanisms underlying inappropriate airway smooth muscle cell growth in asthma remain largely unknown. Myocd has been reported to act as a key transcriptional coactivator in promoting airway-specific smooth muscle development in fetal lungs. Whether Myocd controls airway smooth muscle remodeling in asthma has not been investigated. Mice with lung mesenchyme-specific deletion of <i>Myocd</i> after lung development were generated, and a chronic asthma model was established by sensitizing and challenging the mice with ovalbumin for a prolonged period. Comparison of the asthmatic pathology between the <i>Myocd</i> knockout mice and the wild-type controls revealed that abrogation of Myocd mitigated airway smooth muscle cell hypertrophy and hyperplasia, accompanied by reduced peri-airway inflammation, decreased fibrillar collagen deposition on airway walls, and attenuation of abnormal mucin production in airway epithelial cells. Our study indicates that Myocd is a key transcriptional coactivator involved in asthma airway remodeling. Inhibition of Myocd in asthmatic airways may be an effective approach to breaking the vicious cycle of asthmatic progression, providing a novel strategy in treating severe and persistent asthma. © 2022 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"259 3","pages":"331-341"},"PeriodicalIF":5.6000,"publicationDate":"2022-12-09","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://pathsocjournals.onlinelibrary.wiley.com/doi/epdf/10.1002/path.6044","citationCount":"1","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6044","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 1
引用
批量引用
Abstract
Abnormal growth of airway smooth muscle cells is one of the key features in asthmatic airway remodeling, which is associated with asthma severity. The mechanisms underlying inappropriate airway smooth muscle cell growth in asthma remain largely unknown. Myocd has been reported to act as a key transcriptional coactivator in promoting airway-specific smooth muscle development in fetal lungs. Whether Myocd controls airway smooth muscle remodeling in asthma has not been investigated. Mice with lung mesenchyme-specific deletion of Myocd after lung development were generated, and a chronic asthma model was established by sensitizing and challenging the mice with ovalbumin for a prolonged period. Comparison of the asthmatic pathology between the Myocd knockout mice and the wild-type controls revealed that abrogation of Myocd mitigated airway smooth muscle cell hypertrophy and hyperplasia, accompanied by reduced peri-airway inflammation, decreased fibrillar collagen deposition on airway walls, and attenuation of abnormal mucin production in airway epithelial cells. Our study indicates that Myocd is a key transcriptional coactivator involved in asthma airway remodeling. Inhibition of Myocd in asthmatic airways may be an effective approach to breaking the vicious cycle of asthmatic progression, providing a novel strategy in treating severe and persistent asthma. © 2022 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
心肌调节气道平滑肌细胞重塑对慢性哮喘损伤的响应
气道平滑肌细胞的异常生长是哮喘气道重塑的关键特征之一,与哮喘的严重程度有关。哮喘患者气道平滑肌细胞生长异常的机制在很大程度上尚不清楚。据报道,Myocd在促进胎儿肺部气道特异性平滑肌发育中起着关键的转录辅激活因子的作用。Myocd是否控制哮喘患者的气道平滑肌重塑尚未得到研究。产生了肺发育后肺间充质特异性Myocd缺失的小鼠,并通过用卵清蛋白长期致敏和激发小鼠来建立慢性哮喘模型。Myocd敲除小鼠和野生型对照组之间的哮喘病理学比较显示,Myocd的消除减轻了气道平滑肌细胞肥大和增生,同时减少了气道周围炎症,减少了气道壁上的原纤维胶原沉积,并减轻了气道上皮细胞中异常粘蛋白的产生。我们的研究表明,Myocd是参与哮喘气道重塑的关键转录辅激活因子。抑制哮喘气道中的Myocd可能是打破哮喘进展恶性循环的有效方法,为治疗严重和持续性哮喘提供了一种新的策略。©2022作者。病理学杂志由John Wiley&;代表大不列颠及爱尔兰病理学会的Sons有限公司。
本文章由计算机程序翻译,如有差异,请以英文原文为准。

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


