Synthesis of DNA-Encoded Macrocyclic Peptides via Nitrile-Aminothiol Click Reaction
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
DNA-encoded library (DEL) technology holds exciting potential for discovering novel therapeutic macrocyclic peptides (MPs). Herein, we describe the development of a DEL-compatible peptide macrocyclization method that proceeds via intramolecular click-condensation between 3-(2-cyano-4-pyridyl)-l-alanine (Cpa) and an N-terminal cysteine. Cyclization takes place spontaneously in a buffered aqueous solution and affords the cyclized products in excellent yields. The reaction exhibits a broad substrate scope and can be employed to generate MPs of variable ring size and amino acid composition.
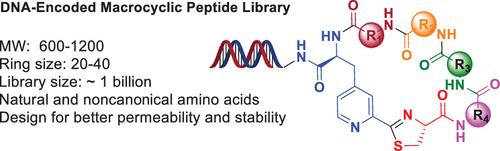
通过腈-氨基硫醇点击反应合成DNA编码的大环肽。
DNA编码文库(DEL)技术在发现新型治疗性大环肽(MP)方面具有令人兴奋的潜力。在此,我们描述了一种DEL兼容肽大环化方法的开发,该方法通过3-(2-氰基-4-吡啶基)-l-丙氨酸(Cpa)和N-末端半胱氨酸之间的分子内点击缩合进行。环化在缓冲水溶液中自发发生,并以优异的产率提供环化产物。该反应显示出广泛的底物范围,并且可以用于产生可变环大小和氨基酸组成的MP。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


