Robust immunity to influenza vaccination in haematopoietic stem cell transplant recipients following reconstitution of humoral and adaptive immunity
Abstract
Objectives
Influenza causes significant morbidity and mortality, especially in high-risk populations. Although current vaccination regimens are the best method to combat annual influenza disease, vaccine efficacy can be low in high-risk groups, such as haematopoietic stem cell transplant (HSCT) recipients.
Methods
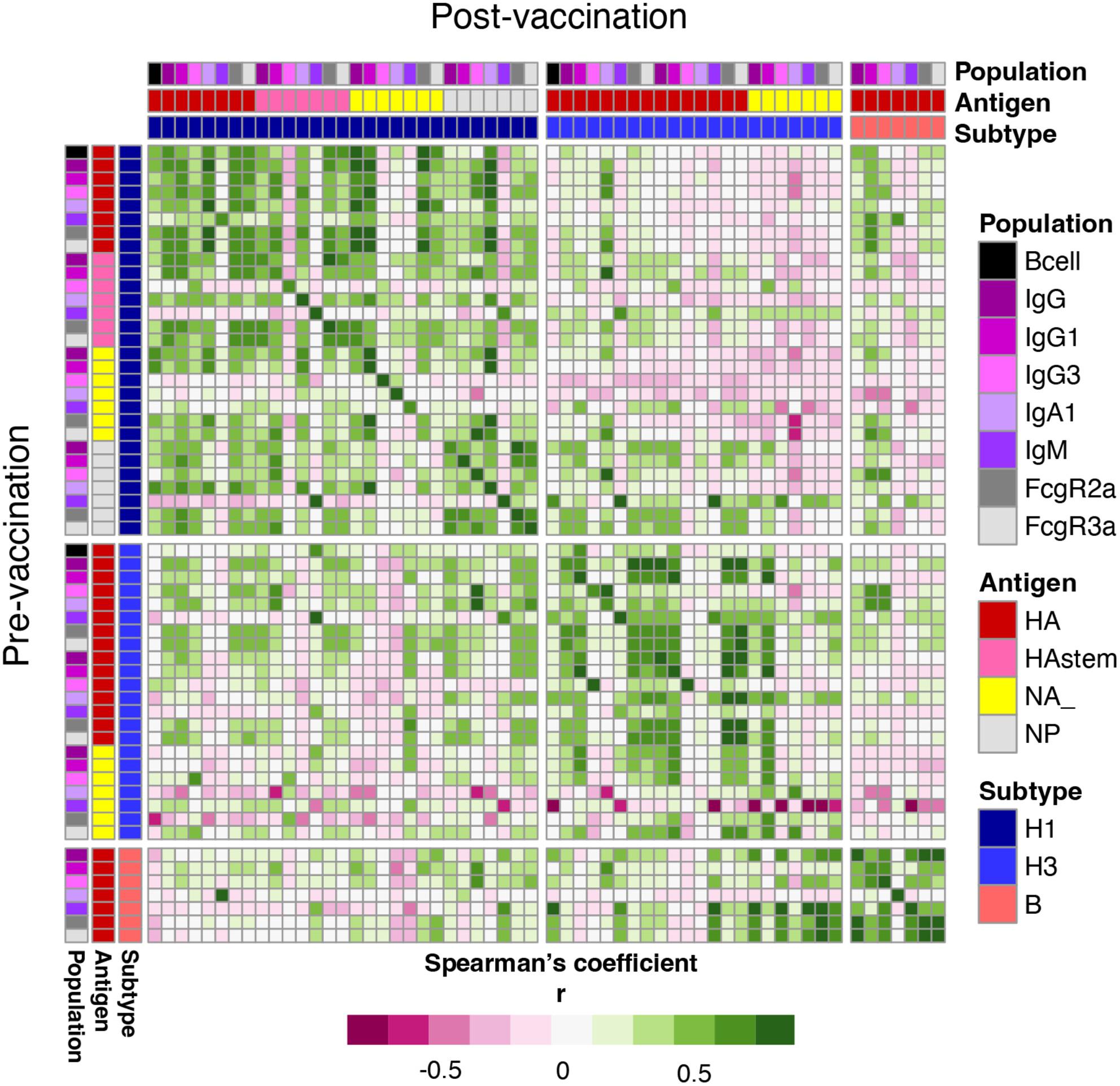
We comprehensively assessed humoral immunity, antibody landscapes, systems serology and influenza-specific B-cell responses, together with their phenotypes and isotypes, to the inactivated influenza vaccine (IIV) in HSCT recipients in comparison to healthy controls.
Results
Inactivated influenza vaccine significantly increased haemagglutination inhibition (HAI) titres in HSCT recipients, similar to healthy controls. Systems serology revealed increased IgG1 and IgG3 antibody levels towards the haemagglutinin (HA) head, but not to neuraminidase, nucleoprotein or HA stem. IIV also increased frequencies of total, IgG class-switched and CD21loCD27+ influenza-specific B cells, determined by HA probes and flow cytometry. Strikingly, 40% of HSCT recipients had markedly higher antibody responses towards A/H3N2 vaccine strain than healthy controls and showed cross-reactivity to antigenically drifted A/H3N2 strains by antibody landscape analysis. These superior humoral responses were associated with a greater time interval after HSCT, while multivariant analyses revealed the importance of pre-existing immune memory. Conversely, in HSCT recipients who did not respond to the first dose, the second IIV dose did not greatly improve their humoral response, although 50% of second-dose patients reached a seroprotective HAI titre for at least one of vaccine strains.
Conclusions
Our study demonstrates efficient, although time-dependent, immune responses to IIV in HSCT recipients, and provides insights into influenza vaccination strategies targeted to immunocompromised high-risk groups.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


