Asymmetric Synthesis of Chiral Primary Amines by Ruthenium-Catalyzed Direct Reductive Amination of Alkyl Aryl Ketones with Ammonium Salts and Molecular H2
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 101
Abstract
A ruthenium/C3-TunePhos catalytic system has been identified for highly efficient direct reductive amination of simple ketones. The strategy makes use of ammonium acetate as the amine source and H2 as the reductant and is a user-friendly and operatively simple access to industrially relevant primary amines. Excellent enantiocontrol (>90% ee for most cases) was achieved with a wide range of alkyl aryl ketones. The practicability of this methodology has been highlighted by scalable synthesis of key intermediates of three drug molecules. Moreover, an improved synthetic route to the optimal diphosphine ligand C3-TunePhos is also presented.
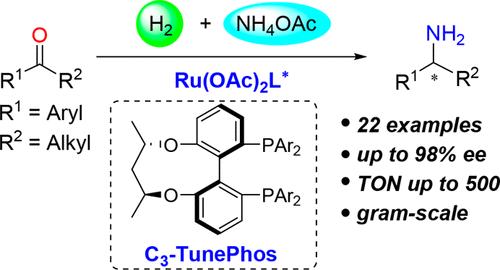
钌催化烷基芳基酮与铵盐和H2直接还原胺化合成手性伯胺的研究
钌/C3-TunePhos催化体系用于简单酮类的高效直接还原胺化反应。该策略以乙酸铵为胺源,H2为还原剂,是一种用户友好且操作简单的工业相关伯胺获取方法。广泛的烷基芳基酮实现了良好的对映控制(大多数情况下90% ee)。这种方法的实用性已经突出了可扩展的合成三种药物分子的关键中间体。此外,还提出了最佳二膦配体C3-TunePhos的改进合成路线。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


