A specific anti-COVID-19 BNT162b2 vaccine-induced early innate immune signature positively correlates with the humoral protective response in healthy and multiple sclerosis vaccine recipients
Abstract
Objectives
The very rapidly approved mRNA-based vaccines against SARS-CoV-2 spike glycoprotein, including Pfizer-BioNTech BNT162b2, are effective in protecting from severe coronavirus disease 2019 (COVID-19) in immunocompetent population. However, establishing the duration and identifying correlates of vaccine-induced protection will be crucial to optimise future immunisation strategies. Here, we studied in healthy vaccine recipients and people with multiple sclerosis (pwMS), undergoing different therapies, the regulation of innate immune response by mRNA vaccination in order to correlate it with the magnitude of vaccine-induced protective humoral responses.
Methods
Healthy subjects (n = 20) and matched pwMS (n = 22) were longitudinally sampled before and after mRNA vaccination. Peripheral blood mononuclear cell (PBMC)-associated type I and II interferon (IFN)-inducible gene expression, serum innate cytokine/chemokine profile as well as binding and neutralising anti-SARS-COV-2 antibodies (Abs) were measured.
Results
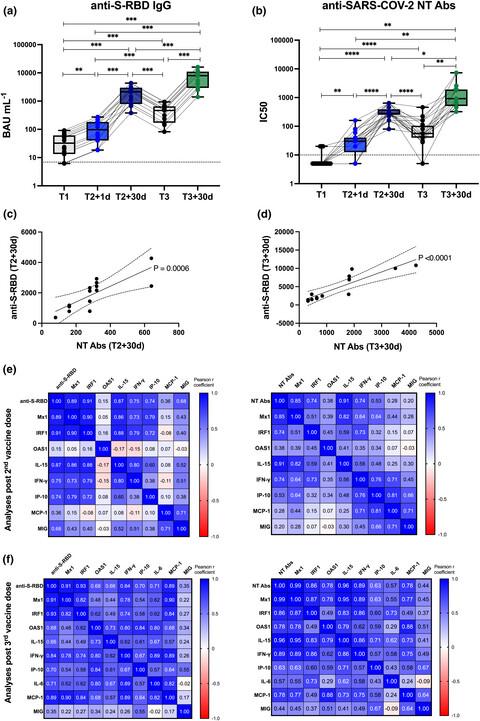
We identified an early immune module composed of the IFN-inducible genes Mx1, OAS1 and IRF1, the serum cytokines IL-15, IL-6, TNF-α and IFN-γ and the chemokines IP-10, MCP-1 and MIG, induced 1 day post second and third BNT162b2 vaccine doses, strongly correlating with magnitude of humoral response to vaccination in healthy and MS vaccinees. Moreover, induction of the early immune module was dramatically affected in pwMS treated with fingolimod and ocrelizumab, both groups unable to induce a protective humoral response to COVID-19 vaccine.
Conclusion
Overall, this study suggests that the vaccine-induced early regulation of innate immunity is mediated by IFN signalling, impacts on the magnitude of adaptive responses and it might be indicative of vaccine-induced humoral protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


